FIRST FORCE- triclosan soap
Basic Packaging Industries Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
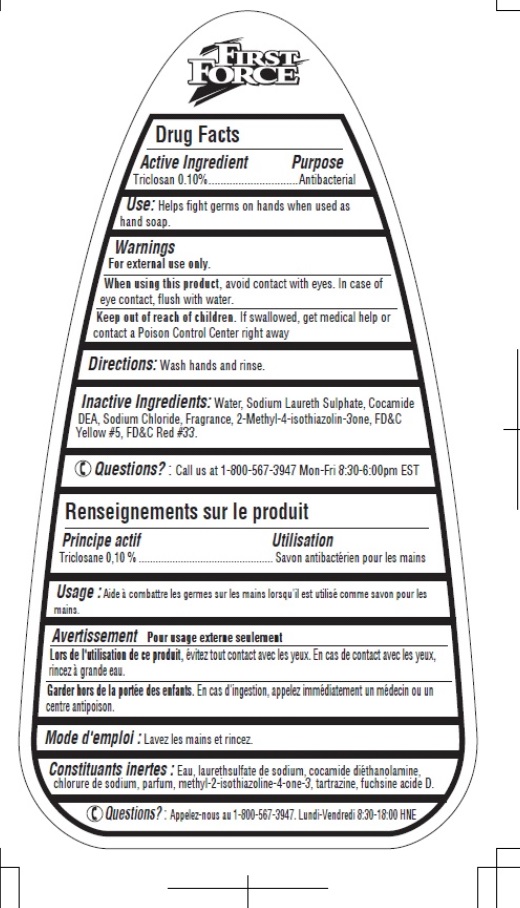
Drug Facts
Inactive Ingredients
Water, Sodium Laureth Sulphate, Cocamide DEA, Sodium Chloride, Fragrance, 2-methyl-4-isothiazolin-3one, FD&C Yellow # 5, FD&C Red #33.
Principal Display Panel
FIRST
FORCE
Gentle on
Hands
Doux pour
les mains
Antibacterial
HANDSOAP
Antibacterien
SAVON POUR LES MAINS
Dish Detergent / Detergent a vaiselle
NO PHOSPHATES / SANS PHOSPHATES
Made in Canada
Fabrique au Canada
Distributed By/Distribue par:
Basic Packaging Industries Inc.
5591 Mc Adam Road
Mississauga, ON. Canada L4Z 1N4
NET 32 FL OZ
(950 mL)

| FIRST FORCE
triclosan soap |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Basic Packaging Industries Inc. (208776468) |
Revised: 7/2017
Document Id: 81015fd2-4e43-4271-a5a0-f40b1ff7f567
Set id: 9eef165d-4810-4d4f-b848-f7c89b681dde
Version: 2
Effective Time: 20170710
Basic Packaging Industries Inc.