LOPERAMIDE HCL- loperamide hcl tablet
L.N.K. International, Inc.
----------
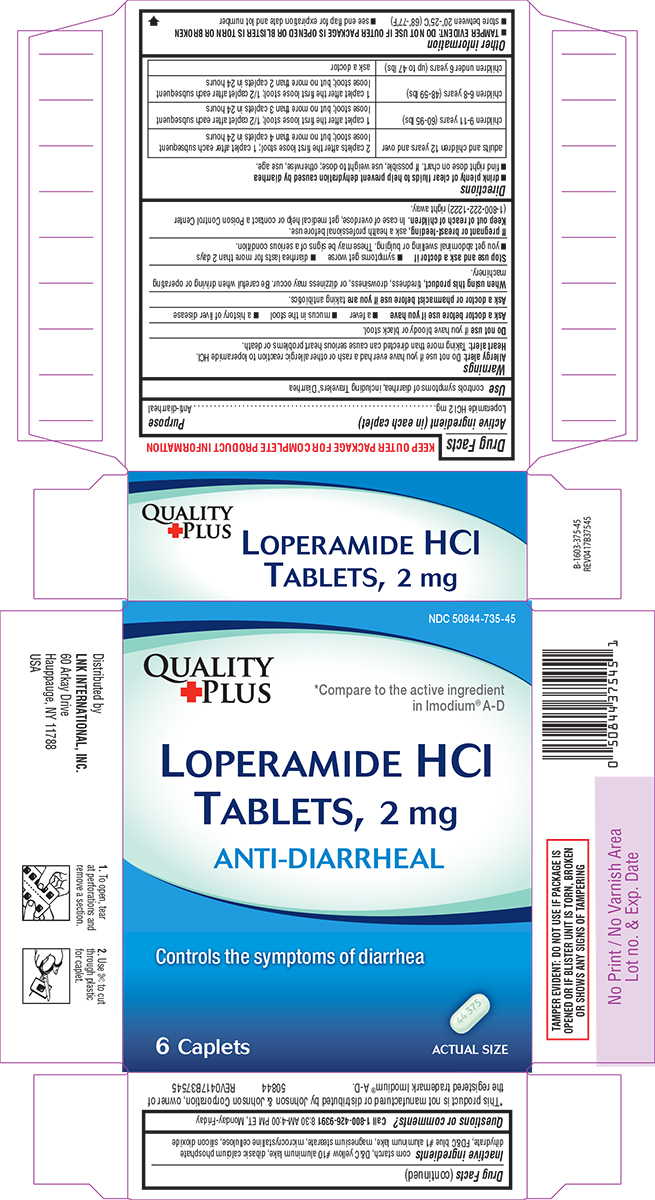
LNK 44-375
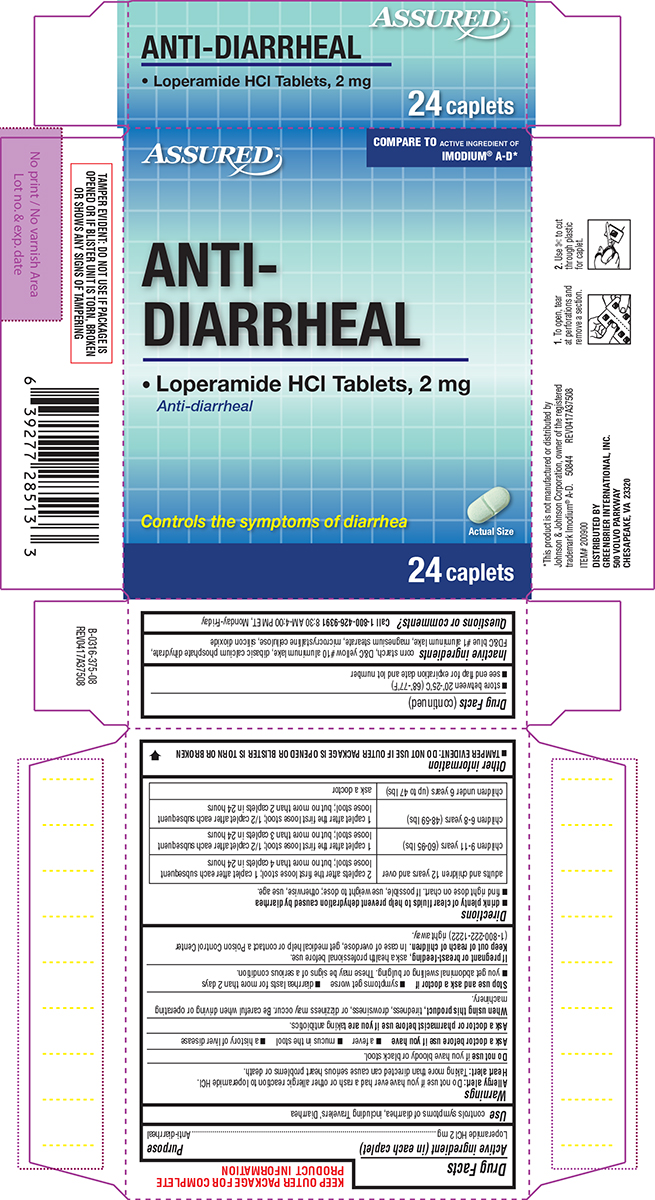
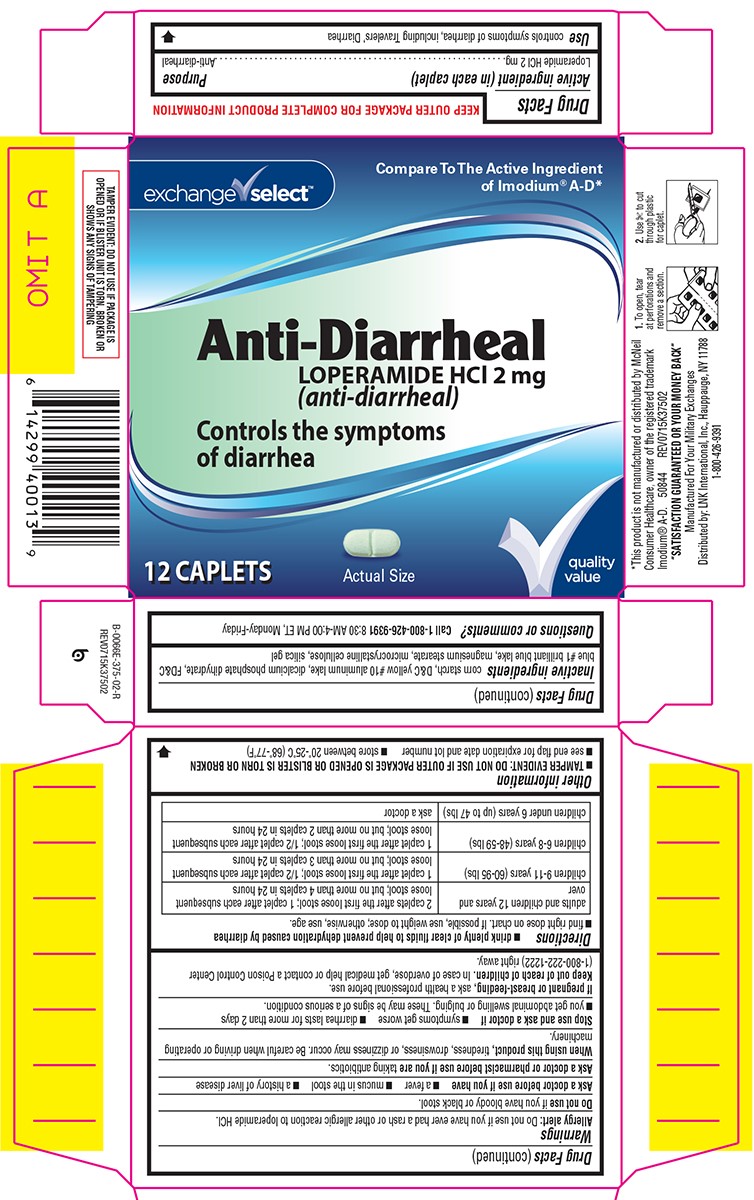
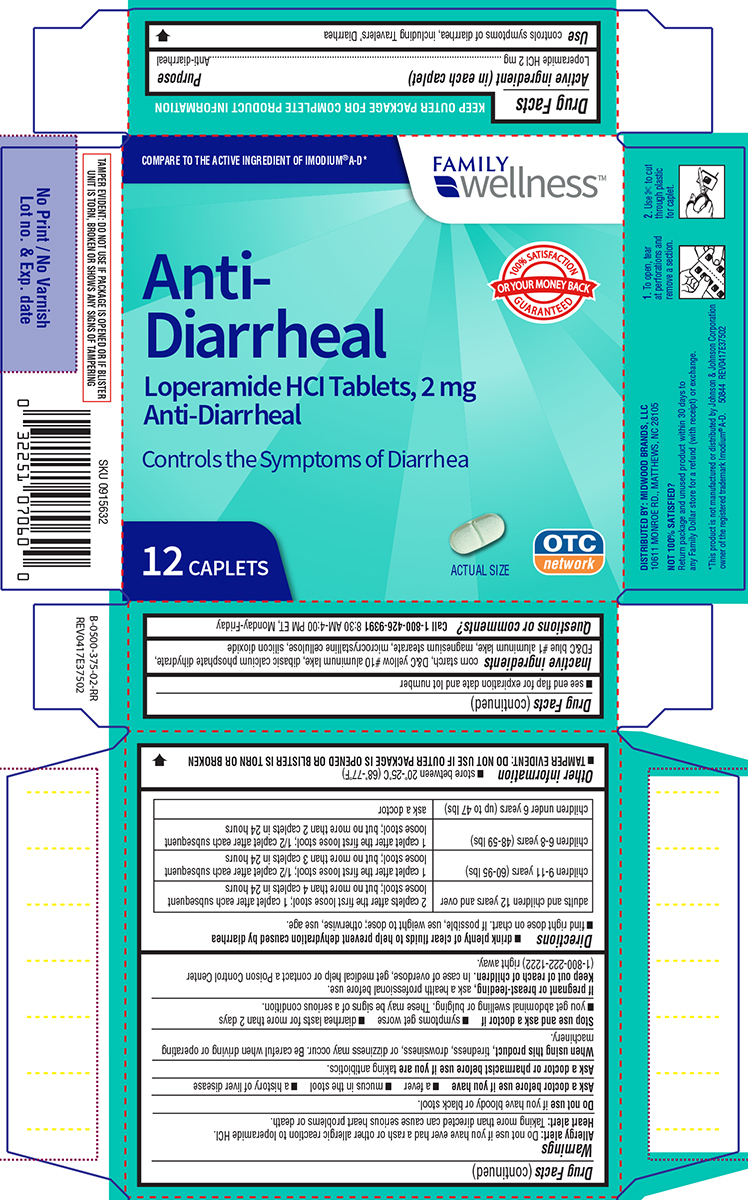
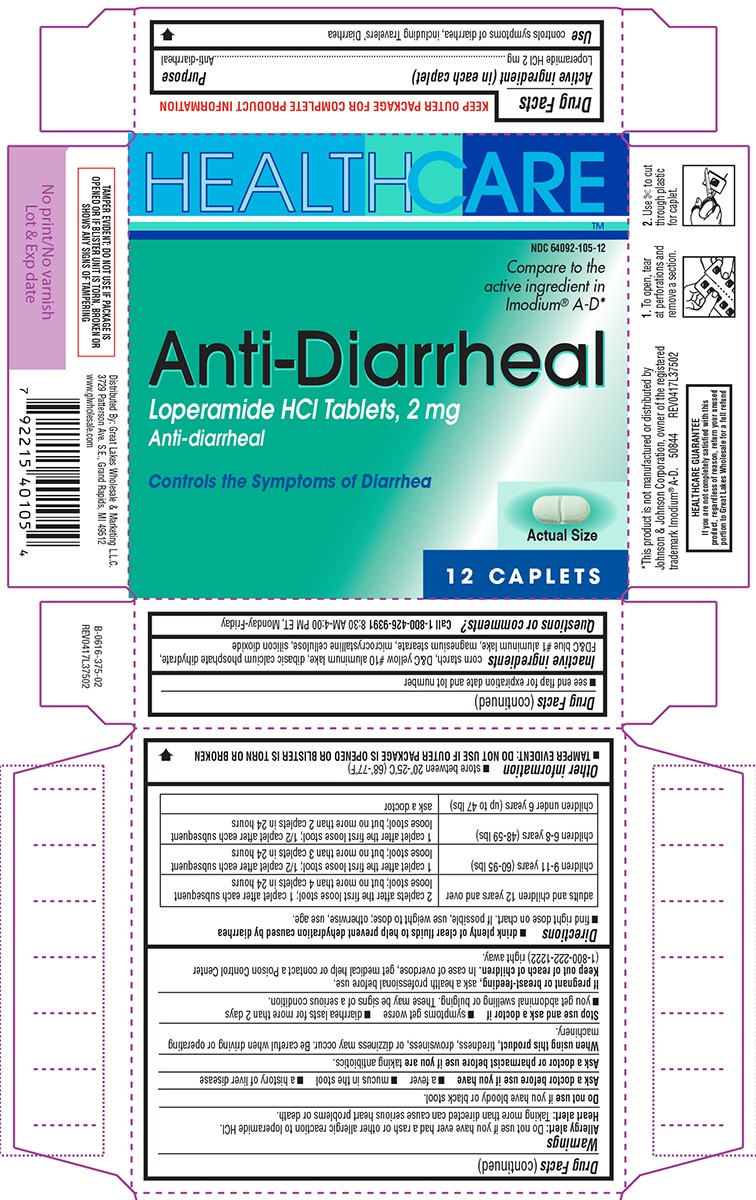
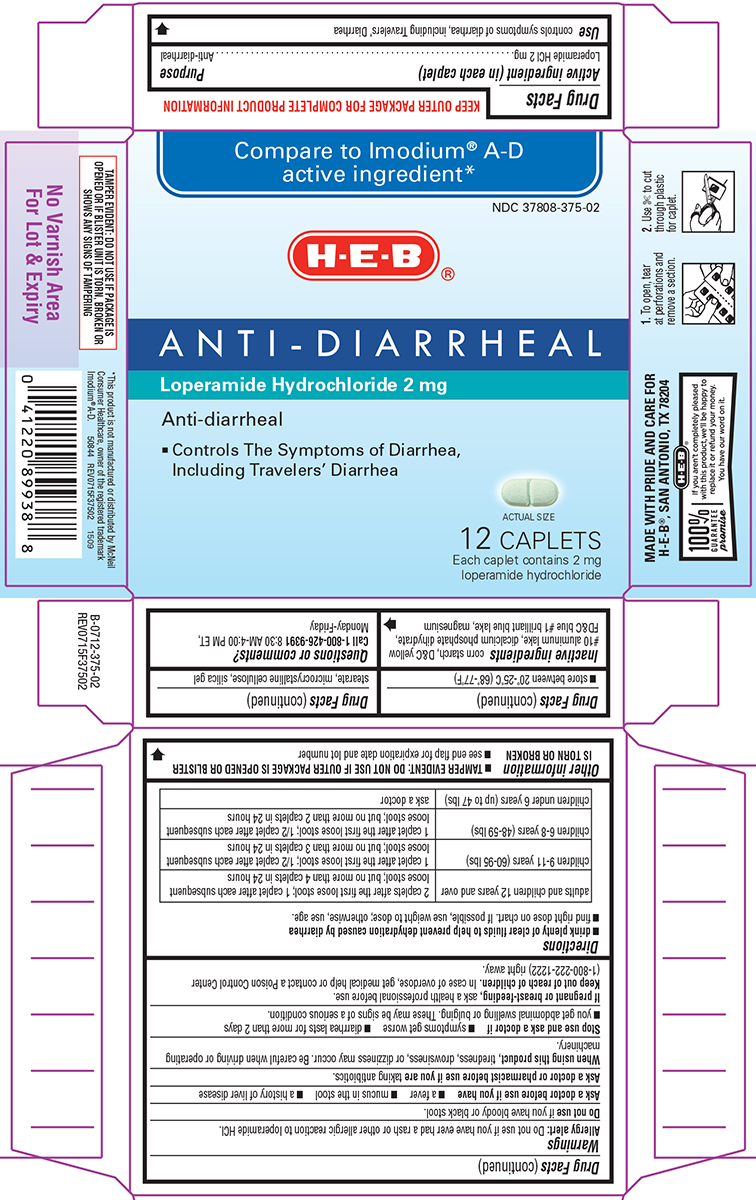
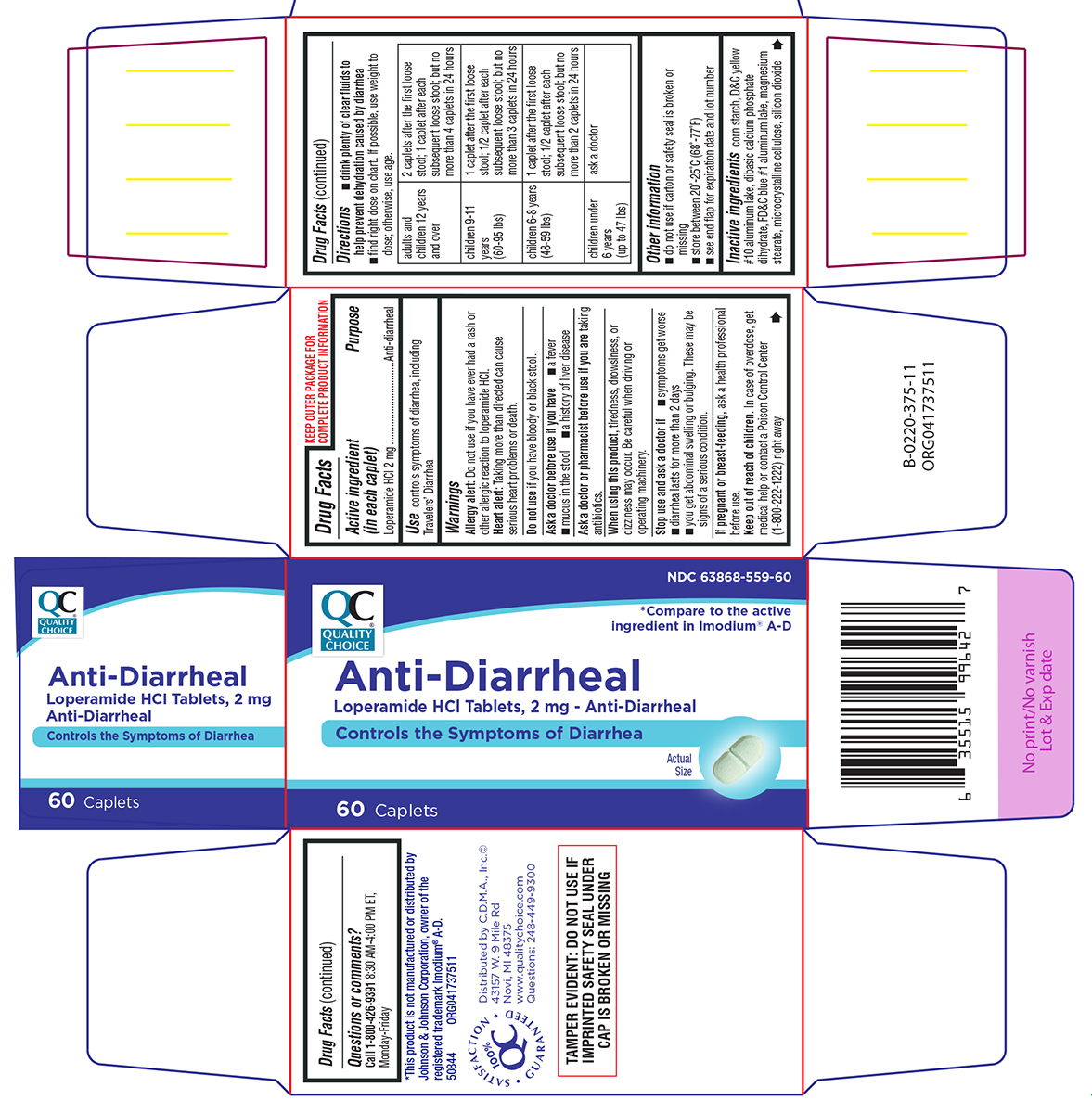
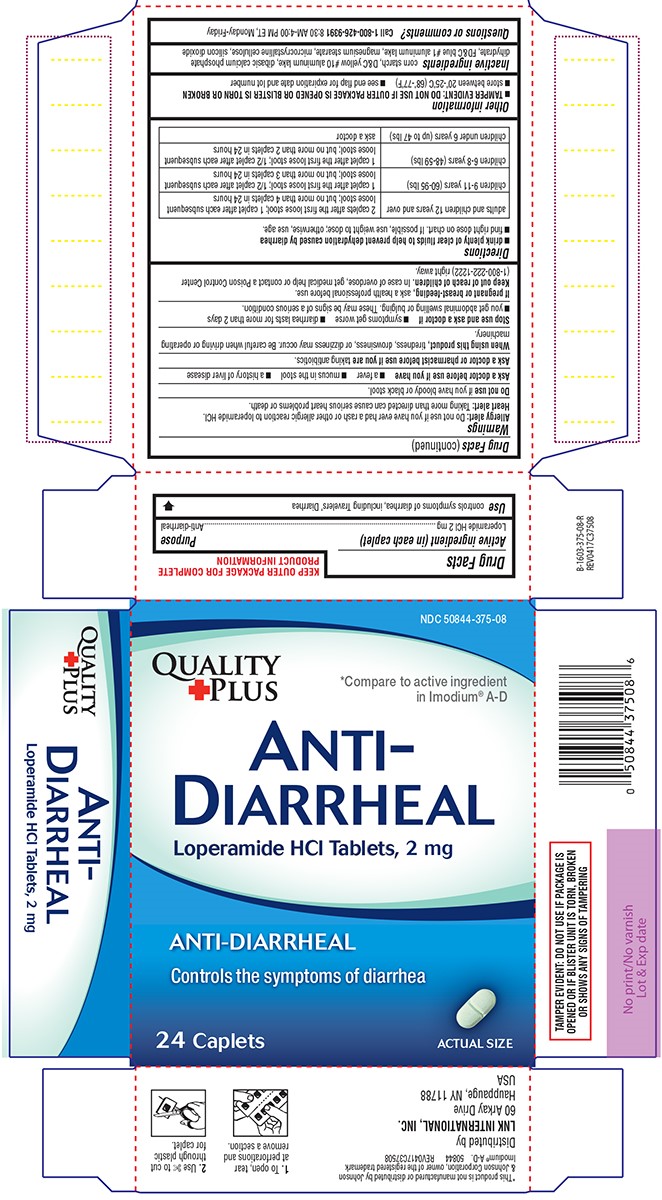
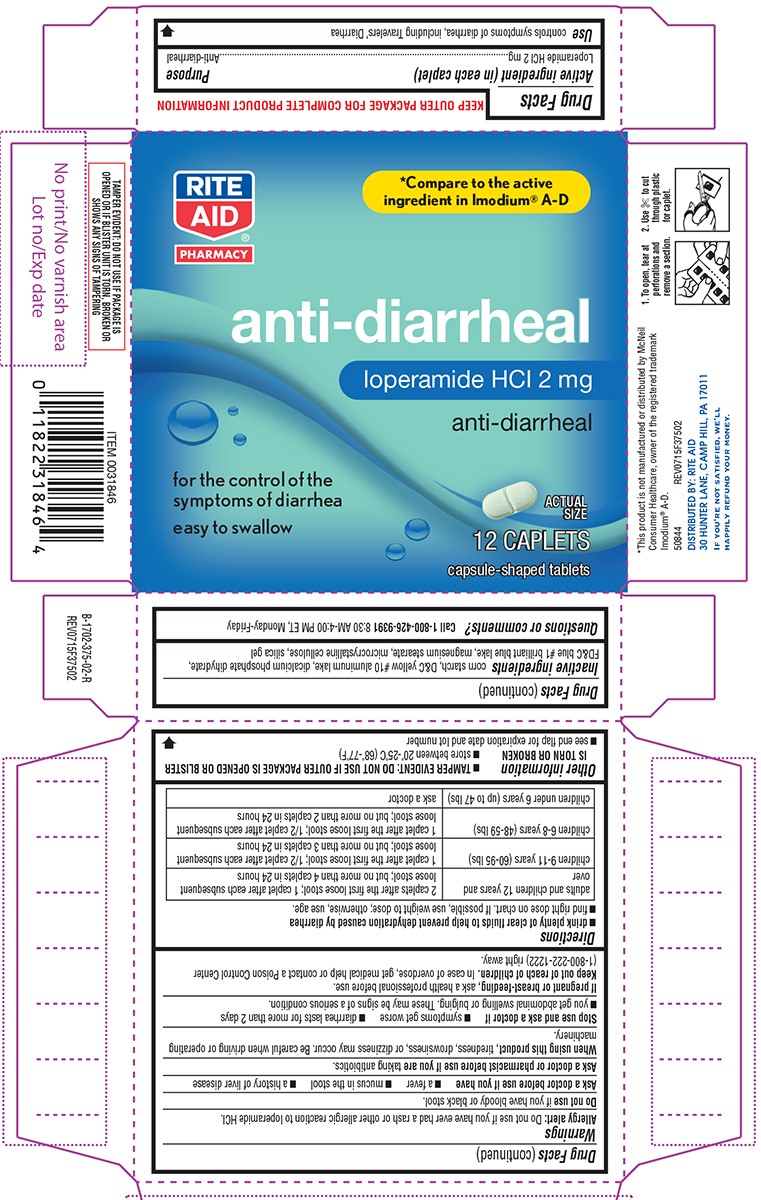
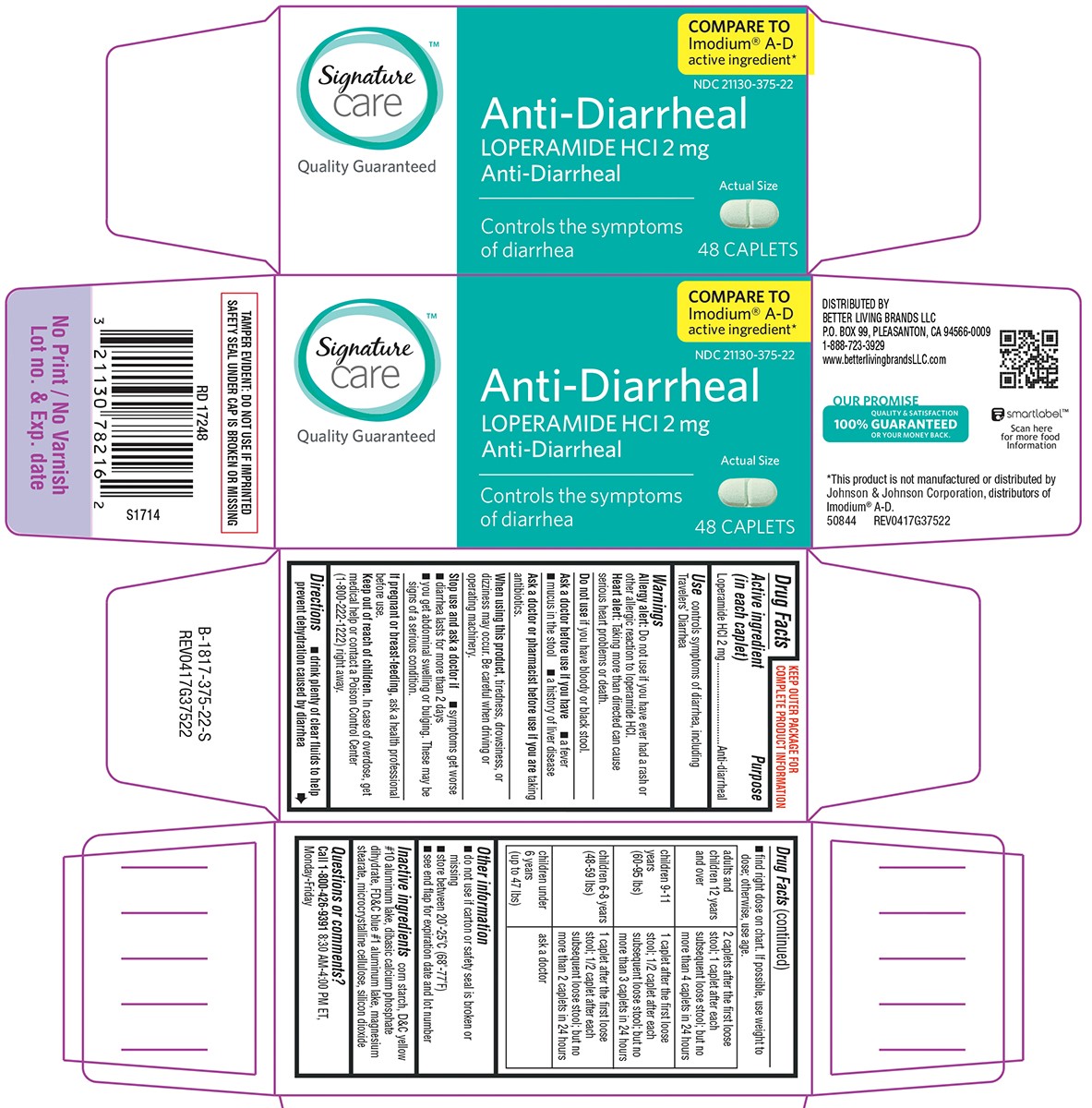
Warnings
Allergy alert: Do not use if you have ever had a rash or other allergic reaction to loperamide HCl.
Heart alert: Taking more than directed can cause serious heart problems or death.
When using this product,
tiredness, drowsiness, or dizziness may occur. Be careful when driving or operating machinery.
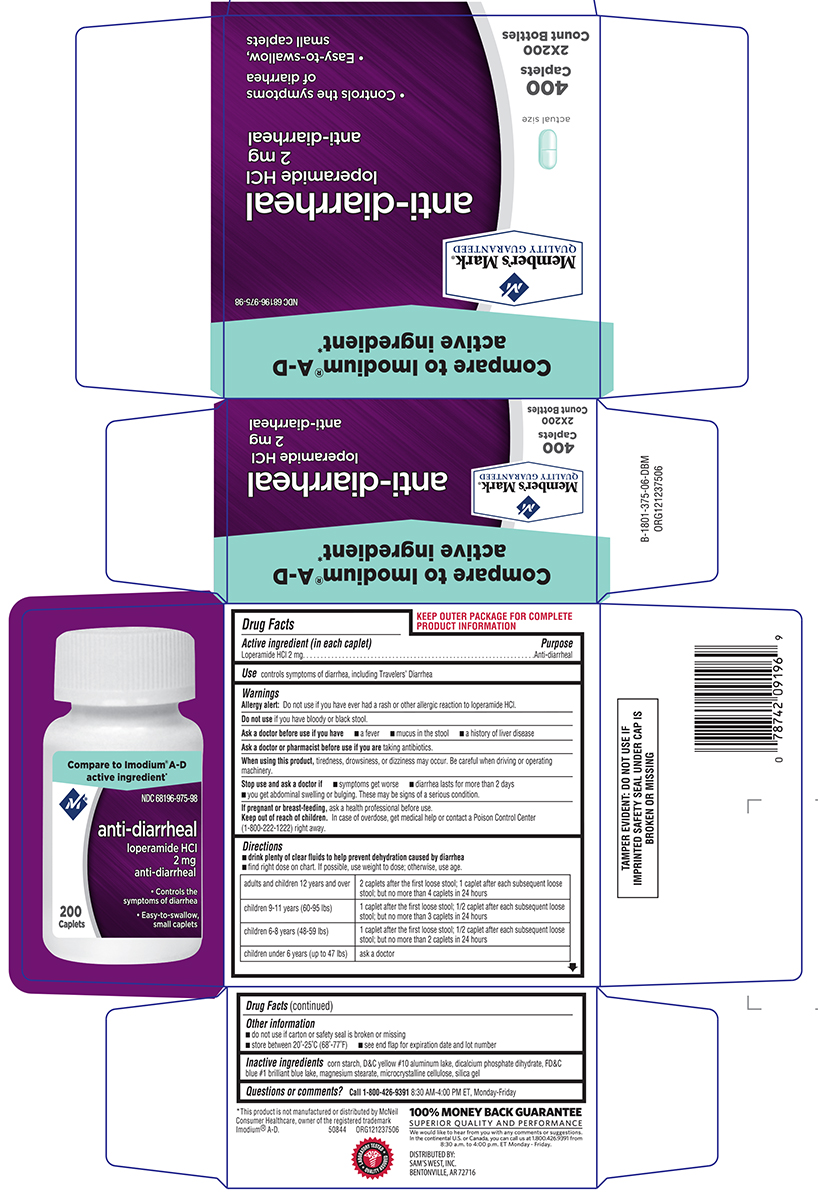
Directions
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
| adults and children 12 years and over |
2 caplets after the first loose stool; 1 caplet after each subsequent loose stool; but no more than 4 caplets in 24 hours |
| children 9-11 years (60-95 lbs) | 1 caplet after the first loose stool; 1/2 caplet after each subsequent loose stool; but no more than 3 caplets in 24 hours |
| children 6-8 years (48-59 lbs) | 1 caplet after the first loose stool; 1/2 caplet after each subsequent loose stool; but no more than 2 caplets in 24 hours |
| children under 6 years (up to 47 lbs) | ask a doctor |
Other information
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store between 20°-25°C (68°-77°F)
- see end flap for expiration date and lot number
| LOPERAMIDE HCL
loperamide hcl tablet |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - L.N.K. International, Inc. (038154464) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | MANUFACTURE(50844-847) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | PACK(50844-847) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | PACK(50844-847) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | PACK(50844-847) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | PACK(50844-847) | |
Revised: 2/2018
Document Id: 2d364740-1278-4c0e-b085-e36e62f35dae
Set id: 9e97d5d7-373e-41d7-afa1-7c57cf2e7b1a
Version: 4
Effective Time: 20180222
L.N.K. International, Inc.