I.V. PREP ANTISEPTIC WIPE- isopropyl alcohol solution
Smith & Nephew, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
I.V. Prep Antiseptic Wipe
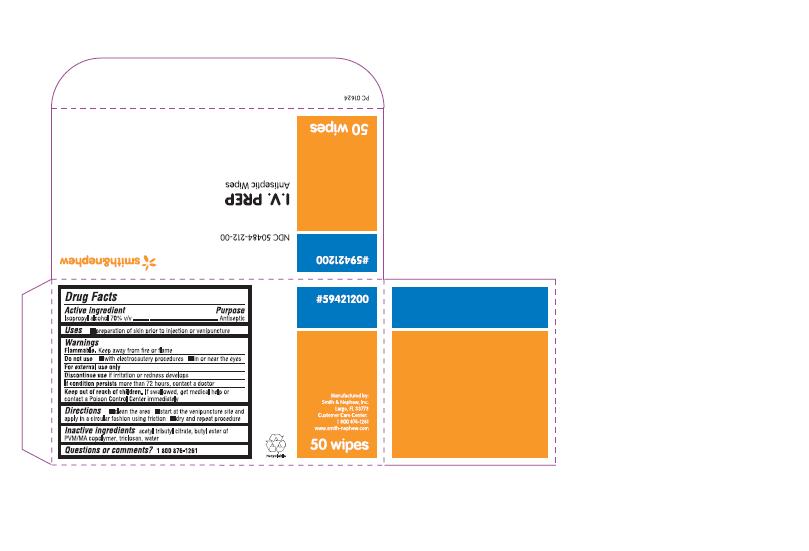
WARNINGS
DIRECTIONS
- clean the area
- start at the venipuncture site and apply in a circular fashion using friction
- dry and repeat procedure
QUESTION OR COMMENTS?
Smith & Nephew, Inc
Largo, FL 33773
Customer Care Center:
1 800 876-1261
www.smith-nephew.com
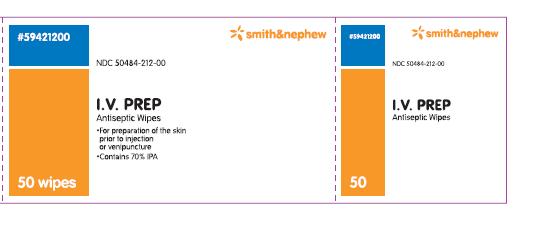
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL- BOX OF 50 WIPES (Front)
#59421200
NDC 50484-212-00
I.V. PREP
Antiseptic Wipes
- for preparation of the skin prior to injection or venipuncture
- contains 70% IPA
50 Wipes
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL- BOX OF 50 WIPES (Back)
#59421200
NDC 50484-212-00
I.V. PREP
Antiseptic Wipes
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL- 1 Packet
#59421200
NDC 50484-212-00
I.V. PREP
Antiseptic Wipes
- for preparation of the skin prior to injection or venipuncture
- contains 70% IPA
1 Wipe

| I.V. PREP ANTISEPTIC WIPE
isopropyl alcohol solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Smith & Nephew, Inc. (827731451) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Span Packaging Services LLC dba Multi-Pack Solutions | 557434805 | MANUFACTURE(50484-212) | |