APRIL BATH AND SHOWER ANTI-BACTERIAL HAND 3 PACK- triclosan
Greenbrier International, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
APRIL Bath & Shower Anti-bacterial Hand Lotions 3 Pack
APRIL Bath and Shower anti-bacterial hand lotion VANILLA BROWN SUGAR SCENTED
APRIL Bath and Shower anti-bacterial hand lotion
JAPANESE BLOSSOM SCENTED
APRIL Bath and Shower
anti-bacterial hand lotion CRANBERRY CHERRY SCENTED
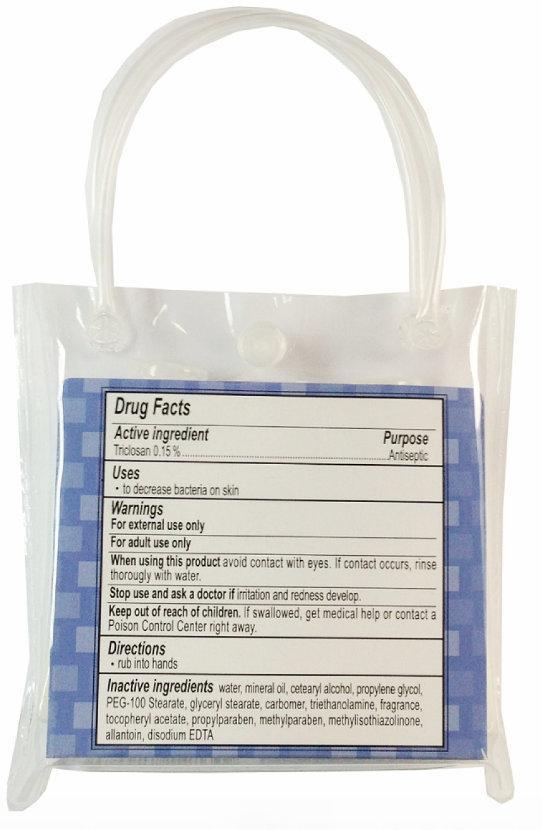
Active Ingredient
Triclosan 0.15%
Uses
- to decrease bacteria on skin
Warnings
For external use only
For adult use only
When using this product avoid contact with eyes. If contact occurs, rinse thoroughly with water.
Stop use and ask a doctor if irritation and redness develop.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Inactive Ingredients
water, mineral oil, cetearyl alcohol, propylene glycol, PEG-100 Stearate, glyceryl stearate, carbomer, triethanolamine, fragrance, tocopheryl acetate, propylparaben, methylparaben, methylisothiazolinone, allantoin, disodium EDTA
APRIL Bath and Shower Anti-bacterial Hand Lotions 3 Pack (33992-0010-3)
APRIL Bath and Shower anti-bacterial hand lotion VANILLA BROWN SUGAR
SCENTED 1oz/28.4g (33992-0011-0)
APRIL Bath and Shower anti-bacterial hand
lotion JAPANESE BLOSSOM SCENTED 1oz/28.4g (33992-0012-0)
APRIL Bath and
Shower anti-bacterial hand lotion CRANBERRY CHERRY SCENTED 1oz/28.4g
(33992-0013-0)


Greenbrier International, Inc.


