SORE THROAT MENTHOL- phenol liquid
QUALITY CHOICE (Chain Drug Marketing Association)
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
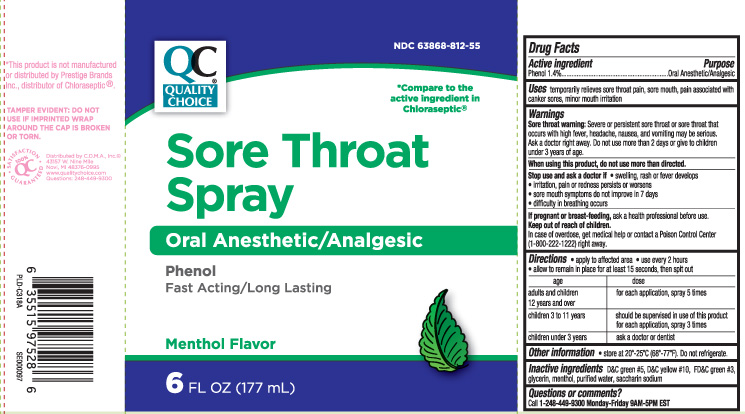
Drug Facts
Uses
temporarily relieves sore throat pain, sore mouth, pain associated with canker sores, minor mouth irritation
Warnings
Sore throat warning: Severe or persistent sore throat or sore throat that occurs with high fever, headache, nausea, and vomiting may be serious. Ask a doctor right away. Do not use more than 2 days or give to children under 3 years of age.
Directions
- apply to the affected area
- use every 2 hours
- allow to remain in place for at least 15 seconds, then spit out
| age | dose |
| adults and children 12 years and over | for each application, spray 5 times |
| children 3 to 11 years | should be supervised in use of this product for each application, spray 3 times |
| children under 3 years | ask a doctor or dentist |
Inactive ingredients
D&C green #5, D&C yellow #10, FD&C green #3, glycerin, menthol, purified water, saccharin sodium
Principal Display Panel
*Compare to the Active Ingredient in Chloraseptic®
Sore Throat Spray
Oral anesthetic/analgesic
Phenol
Fast Acting/Long lasting
Menthol flavor
FL OZ (mL)
*This product is not manufactured or distributed by Prestige Brands Inc., distributor of Chloraseptic®.
TAMPER EVIDENT: DO NOT USE IF IMPRINTED WRAP AROUND THE CAP IS BROKEN OR TORN.
Distributed by C.D.M.A Inc.©
43157 W.Nine Mile
Novi, MI 48376-0995
qualitychoice.com
| SORE THROAT
MENTHOL
phenol liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - QUALITY CHOICE (Chain Drug Marketing Association) (011920774) |