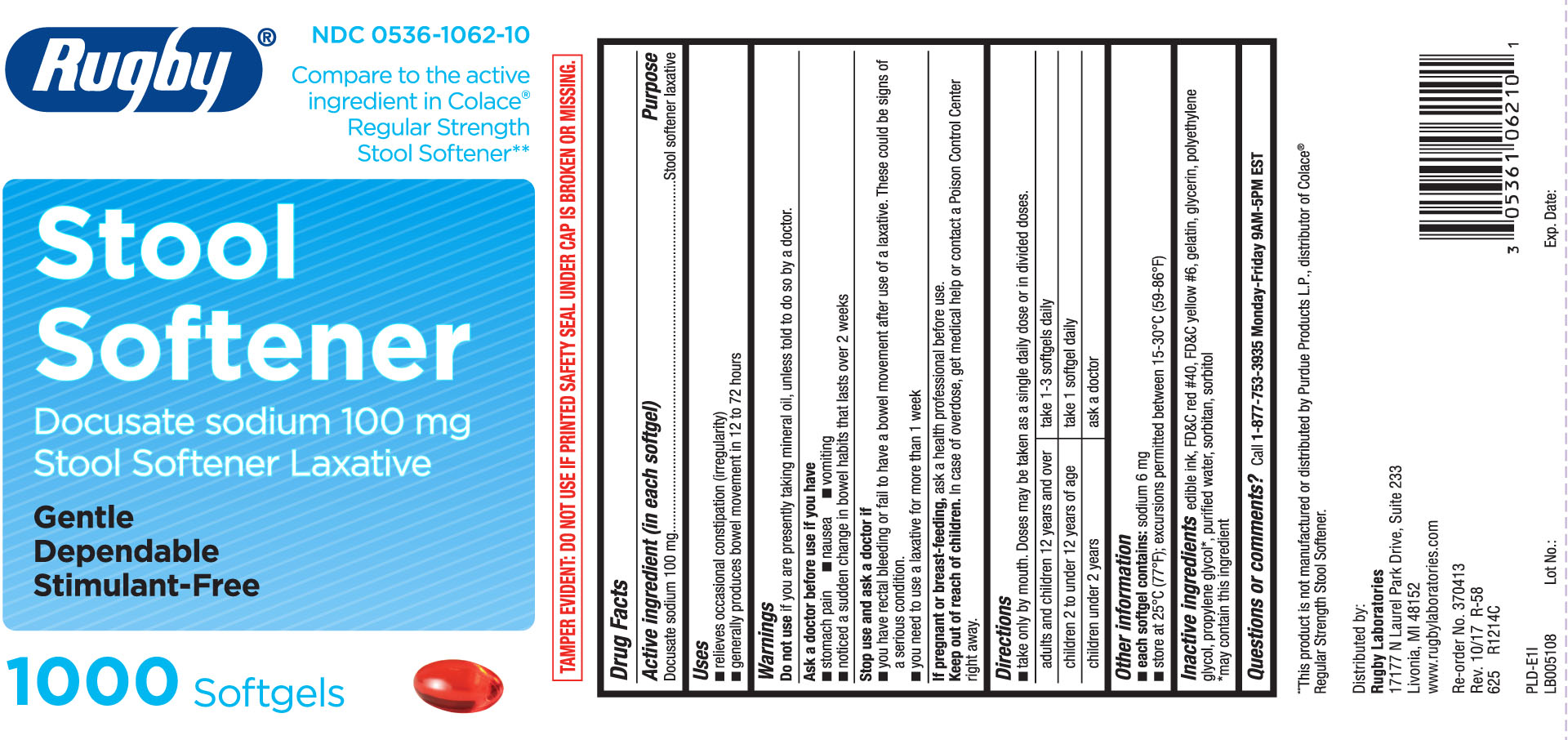
STOOL SOFTENER- docusate sodium capsule, liquid filled
Rugby Laboratories, Inc.
----------
Drug Facts
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 12 to 72 hours
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
Directions
- take only by mouth. Doses may be taken as a single daily dose or in divided doses.
| adults and children 12 years and over | take 1-3 softgels daily |
| children 2 to under 12 years of age | take 1 softgel daily |
| children under 2 years | ask a doctor |
Other information
- each softgel contains: sodium 6 mg
- store at 25ºC (77F); excursions permitted between 15-30ºC (59-86ºF)
Inactive Ingredients
edible ink, FD &C red #40, FD &C yellow #6, gelatin, glycerin, polyethylene glycol, propylene glycol, purified water, sorbitan, sorbitol
*may contains this ingredients
Principal Display Panel
Compare to the active ingredient in Colace® Regular Strength Stool Softener**
Stool Softener
Docusate sodium 100mg
Stool Softener Laxative
Gentle
Effective
Stimulant-Free
Softgels
**This product is not manufactured or distributed by Purdue Product L.P., distributor of Colace® Regular Strength Stool Softener.
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BEOKEN OR MISSING.
Distributed by:
Rugby Laboratories
17177 N Laurel Park Drive, Suite 233
Livonia, MI 48150
www.rugbylaboratories.com
| STOOL SOFTENER
docusate sodium capsule, liquid filled |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Rugby Laboratories, Inc. (079246066) |