MENSTRUAL CRAMPS- magnesium phosphate, dibasic trihydrate, citrullus colocynthis fruit pulp, black cohosh, and pulsatilla vulgaris tablet, soluble
Hyland's Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
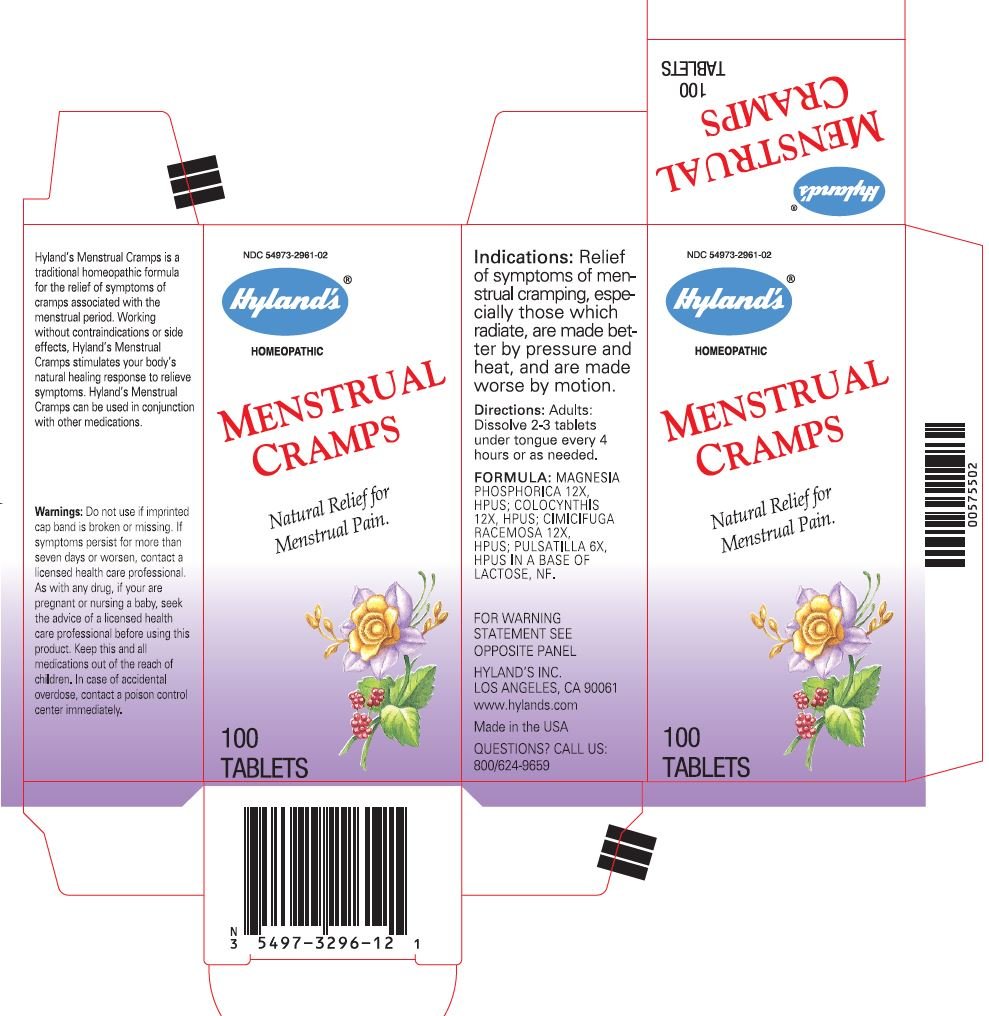
Hyland's Menstrual Cramps is a traditional homeopathic formula for the relief of symptoms of cramps associated with the menstrual period. Working without contraindications or side effects, Hyland's Menstrual Cramps stimulates your body's natural healing response to relieve symptoms. Hyland's Menstrual Cramps can be used in conjunction with other medications.
Warnings
Do not use if imprinted cap band is broken or missing.
If symptoms persist for more than seven days or worsen, contact a licensed health care professional.
As with any drug, if your are pregnant or nursing a baby, seek the advice of a licensed health care professional before using this product.
Keep this and all medications out of the reach of children. In case of accidental overdose, contact a poison control center immediately.
Indications
Relief of symptoms of menstrual cramping, especially those which radiate, are made better by pressure and heat, and are made worse by motion.
Directions
Adults: Dissolve 2-3 tablets under tongue every 4 hours or as needed.
FORMULA
MAGNESIA PHOSPHORICA 12X, HPUS; COLOCYNTHIS 12X, HPUS; CIMICIFUGA RACEMOSA 12X, HPUS; PULSATILLA 6X, HPUS
IN A BASE OF LACTOSE, NF.
HYLAND'S INC.
LOS ANGELES, CA 90061
QUESTIONS?
CALL US: 800/624-9659
PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Carton
NDC 54973-2961-02
Hyland's®
HOMEOPATHIC
MENSTRUAL
CRAMPS
Natural Relief for
Menstrual Pain.
100
TABLETS
Hyland's Inc.
