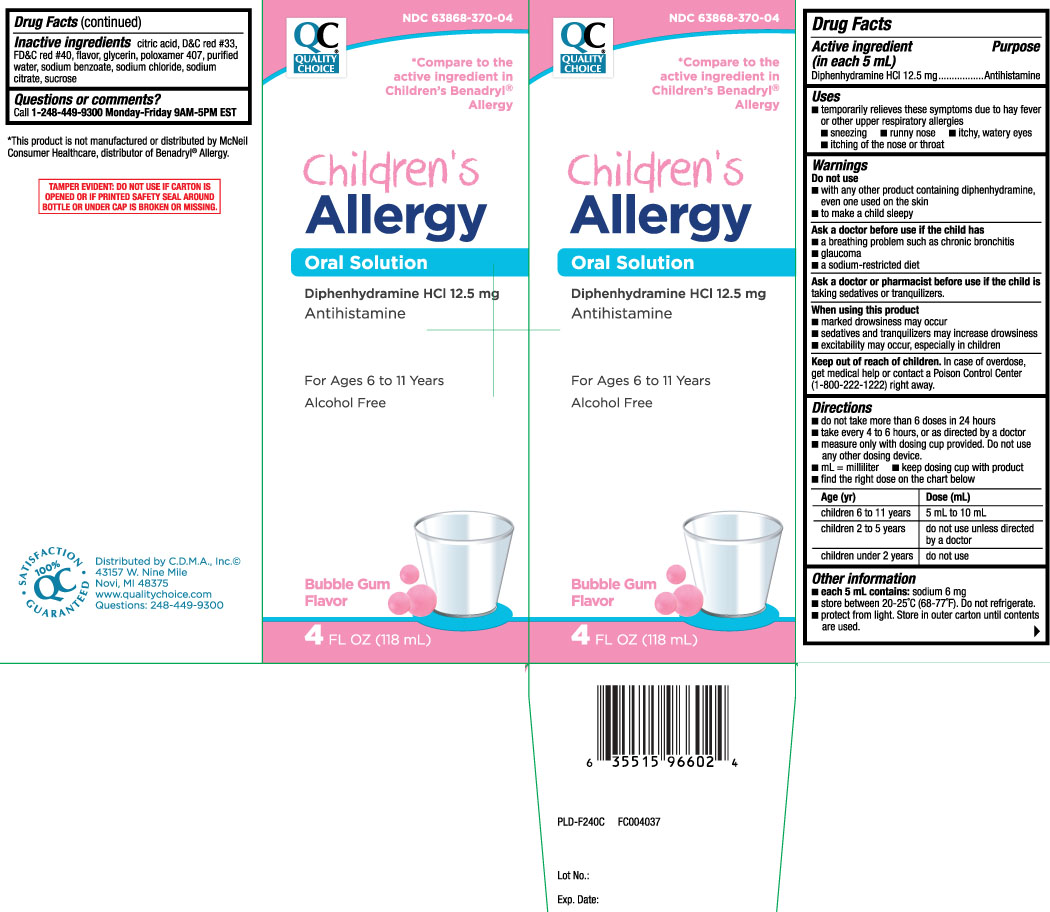
Label: CHILDRENS ALLERGY- diphenhydramine hydrochloride liquid
- NDC Code(s): 63868-370-04
- Packager: QUALITY CHOICE (Chain Drug Marketing Association)
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each 5 mL)
- Purpose
- Uses
-
Warnings
Do not use
- to make a child sleep
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if the child has
- glaucoma
- a breathing problem such as chronic bronchitis
- a sodium-restricted diet
-
Directions
- do not take more than 6 doses in 24 hours
- take every 4 to 6 hours, or as directed by a doctor
- measure only with dosing cup provided. Do not use any other dosing device.
- mL = milliliter
- keep dosing cup with product
- find the right dose on the chart below
Age (yr) Dose (mL) children 6 to 11 years 5 mL to 10 mL children 2 to 5 years do not use unless directed by a doctor children under 2 years do not use - Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
*Compare to the active ingredient in Children's Benadryl® Allergy
Children's Allergy
Oral Solution
Diphenhydramine HCl 12.5 mg
Antihistamine
For Ages 6 to 11 Years
Alcohol Free
Bubblegum Flavor
FL OZ (mL)
*This product is not manufactured or distributed by McNeil Consumer Healthcare distributor Benadryl® Allergy.
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF PRINTED SAFETY SEAL AROUND BOTTLE OR UNDER CAP IS BROKEN OR MISSING.
Distributed by C.D.M.A., Inc.©
43157 W. Nine Mile
Novi, MI 48376-0995
www.qualitychoice.com
Questions: 248-449-9300
- Package Label
-
INGREDIENTS AND APPEARANCE
CHILDRENS ALLERGY
diphenhydramine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63868-370 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 12.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) POLOXAMER 407 (UNII: TUF2IVW3M2) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SUCROSE (UNII: C151H8M554) Product Characteristics Color pink Score Shape Size Flavor BUBBLE GUM Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63868-370-04 1 in 1 BOX 05/30/2014 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 05/30/2014 Labeler - QUALITY CHOICE (Chain Drug Marketing Association) (011920774)