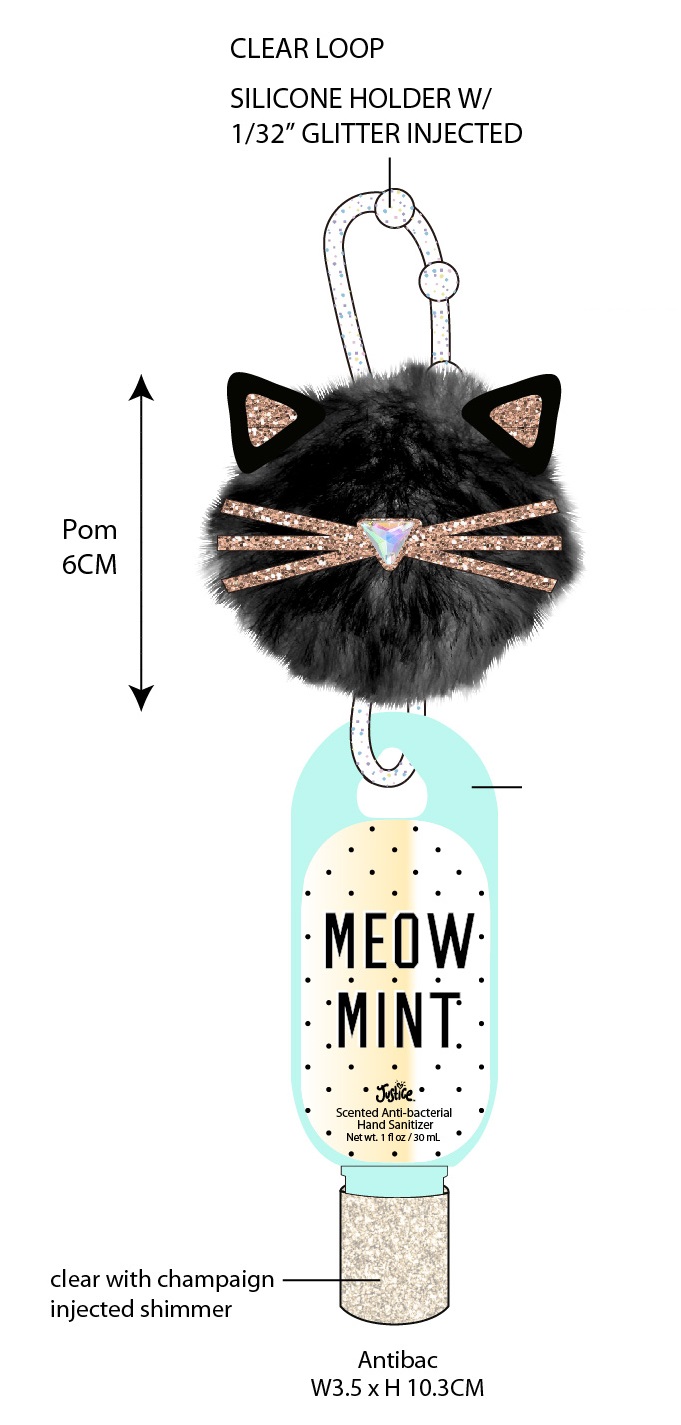
JUSTICE MEOW MINT SCENTED ANTI-BACTERIAL HAND SANITIZER- alcohol gel
Tween Brands, Inc
----------
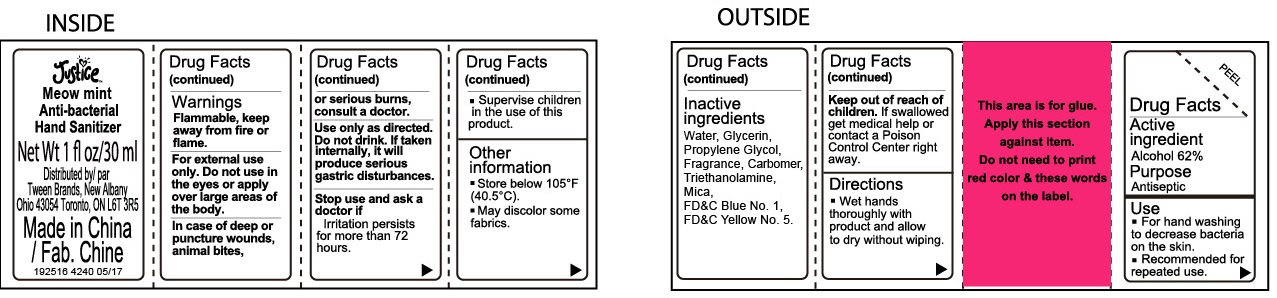
Justice Meow Mint Scented Anti-bacterial Hand Sanitizer
Warnings
Flammable, keep away from fire or flame.
For external use only.
Directions
- Wet hands thoroughly with product and allow to dry without wiping.
- Supervise children in the use of this product.
| JUSTICE MEOW MINT SCENTED ANTI-BACTERIAL HAND SANITIZER
alcohol gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Tween Brands, Inc (965758188) |
Revised: 1/2024
Document Id: 0fc559f9-e283-4d89-e063-6294a90a37c9
Set id: 9bcbe07d-c912-4535-bbf8-2887b74ed53a
Version: 4
Effective Time: 20240125
Tween Brands, Inc