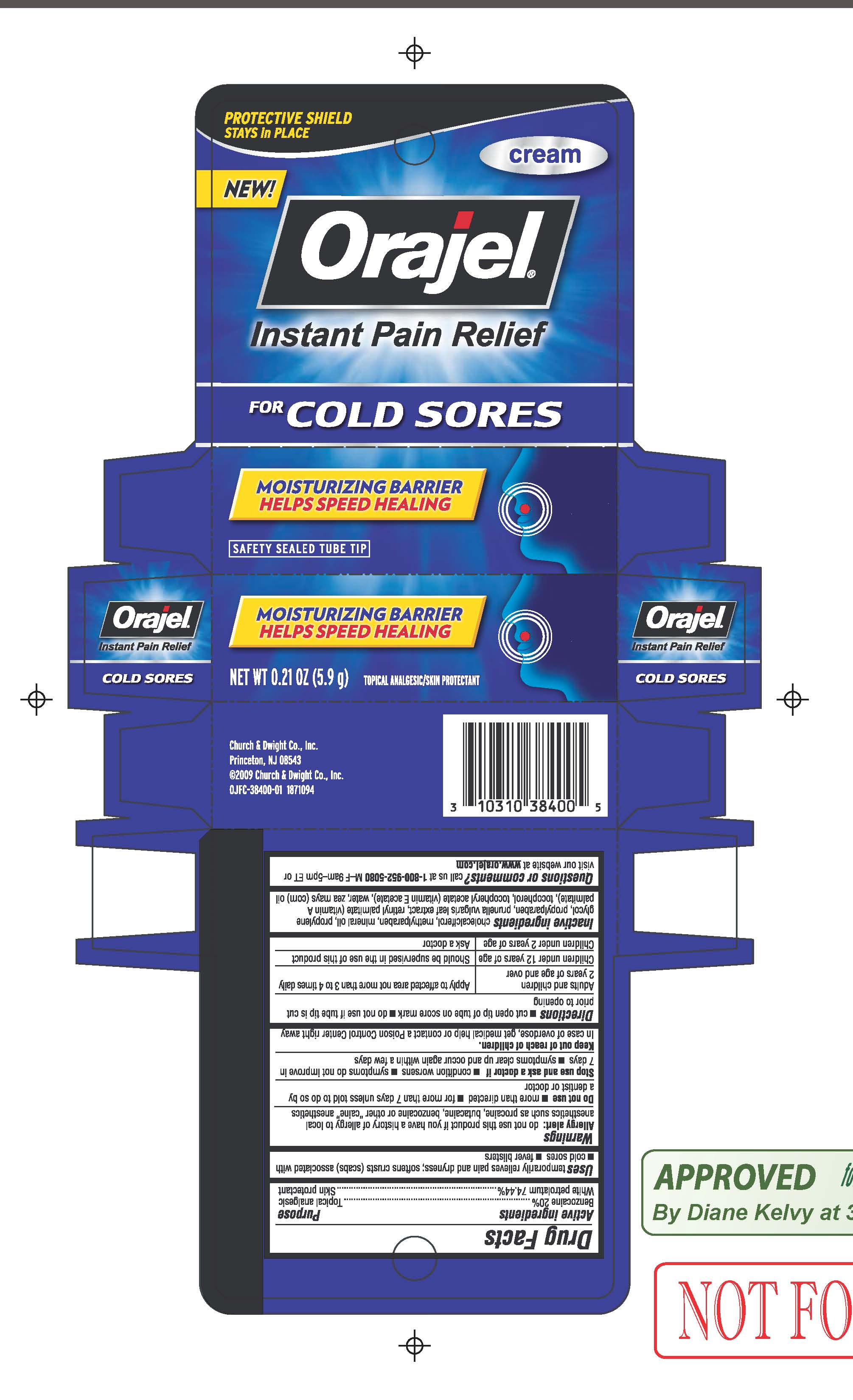
ORAJEL FOR COLD SORES- benzocaine cream
Church & Dwight Co., Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Orajel for Cold Sores
Uses temporarily relieves pain and dryness; softens crusts (scabs) associated with cold sores, fever blisters
Warnings
Allergy alert: do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away
Directions
- cut open tube of tip on score mark
- Do not use if tip is cut prior to opening
Adults and Children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily
Children under 12 years of age: Should be supervised in the use of this product
Children under 2 years of age: Ask a doctor
| ORAJEL FOR COLD SORES
benzocaine cream |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Church & Dwight Co., Inc. (001211952) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Church & Dwight Co., Inc. | 043690812 | manufacture(10237-750) | |