SOOTHE HYDRATION- povidone solution
Bausch & Lomb Incorporated
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
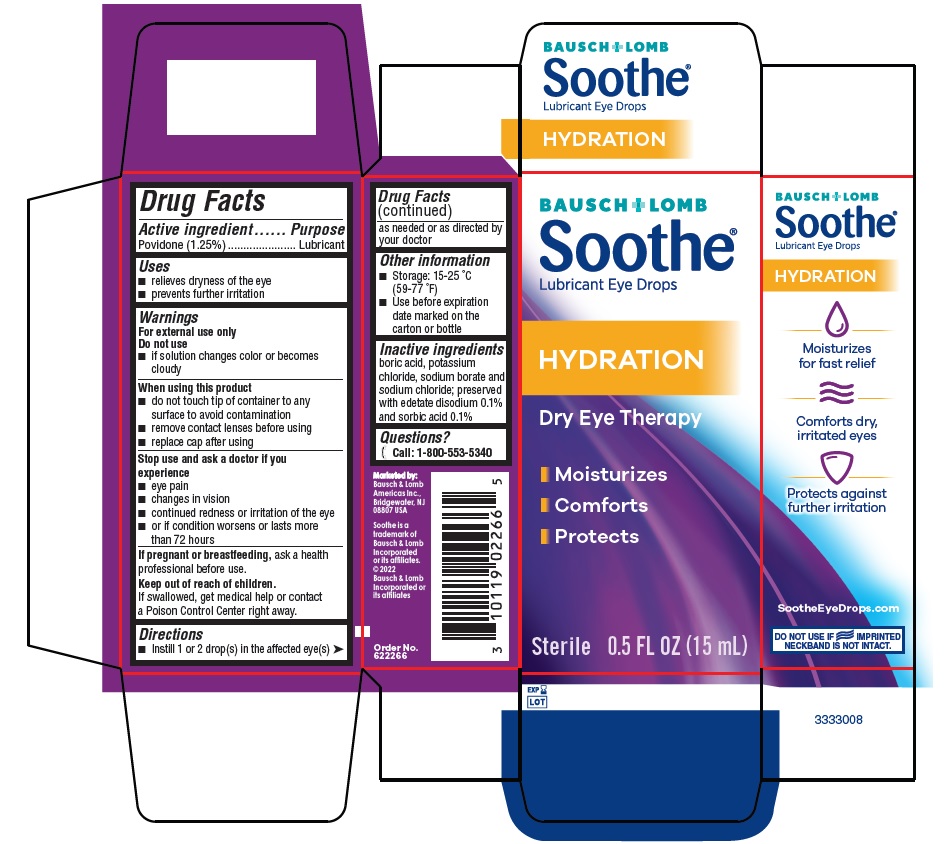
Drug Facts
Warnings
For external use only
Do not use
- •
- if solution changes color or becomes cloudy
When using this product
- •
- do not touch tip of container to any surface to avoid contamination
- •
- remove contact lenses before using
- •
- replace cap after using
Stop use and ask a doctor if you experience
- •
- eye pain
- •
- changes in vision
- •
- continued redness or irritation of the eye
- •
- or if condition worsens or lasts more than 72 hours
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Other information
- •
- Storage: 15-25 °C (59-77 °F)
- •
- Use before expiration date marked on the carton or bottle
| SOOTHE HYDRATION
povidone solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Bausch & Lomb Incorporated (002207751) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bausch & Lomb Incorporated | 079587625 | MANUFACTURE(24208-433) | |
Revised: 2/2023
Document Id: 977450fc-14a0-4bad-b5a5-af98ae25ab06
Set id: 9b6b900e-39c1-4746-a7e7-cb79b6339fcb
Version: 7
Effective Time: 20230221
Bausch & Lomb Incorporated