Label: SEVOFLURANE BOSTONBIO- sevoflurane inhalant
-
Contains inactivated NDC Code(s)
NDC Code(s): 72163-005-25 - Packager: Boston Biopharma
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 9, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
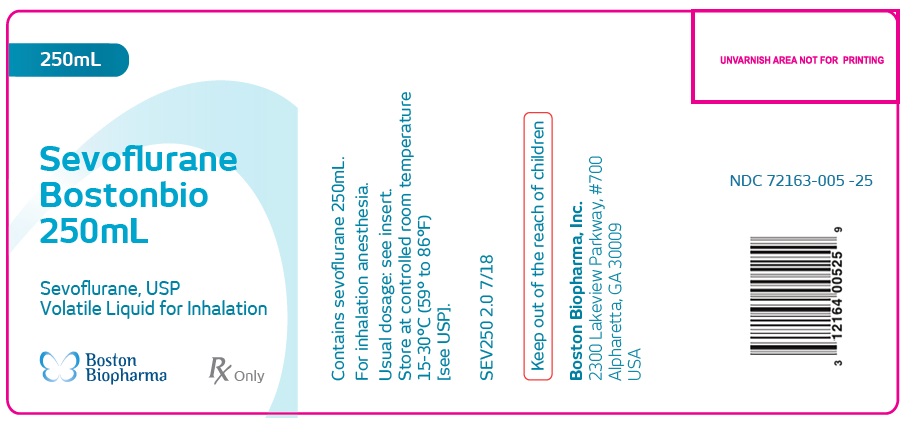
HOW SUPPLIED
Sevoflurane
Bostonbio
250mL
Sevoflurane, USP
Volatile Liquid for InhalationContains sevoflurane 250mL.
For inhalation anesthesia.
Usual dosage: see insert.
Store at controlled room temperature
15-30°C (59° to 86°F)
[see USP].
SEV250 2.0 7/18Keep out of the reach of children
Boston Biopharma, Inc.
2300 Lakeview Parkway, #700
Alpharetta, GA 30009
USANDC 72163-005 -25
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SEVOFLURANE BOSTONBIO
sevoflurane inhalantProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72163-005 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SEVOFLURANE (UNII: 38LVP0K73A) (SEVOFLURANE - UNII:38LVP0K73A) SEVOFLURANE 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72163-005-25 250 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 03/27/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078650 03/27/2020 Labeler - Boston Biopharma (081073771) Registrant - Halocarbon Life Sciences, LLC (109112409) Establishment Name Address ID/FEI Business Operations Pharmasol Corporation 065144289 manufacture(72163-005) , analysis(72163-005) , pack(72163-005) , label(72163-005) Establishment Name Address ID/FEI Business Operations Boston Biopharma 081073771 relabel(72163-005) Establishment Name Address ID/FEI Business Operations Halocarbon Life Sciences, LLC 109112409 api manufacture(72163-005)