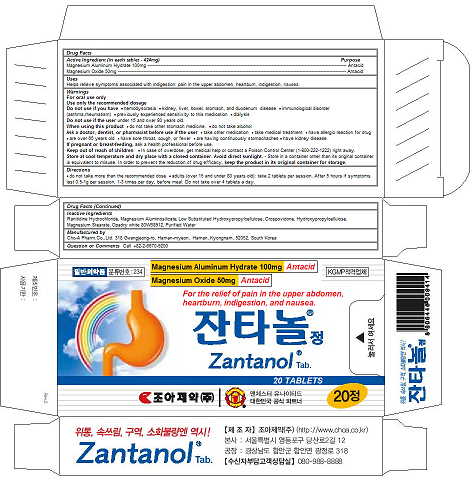
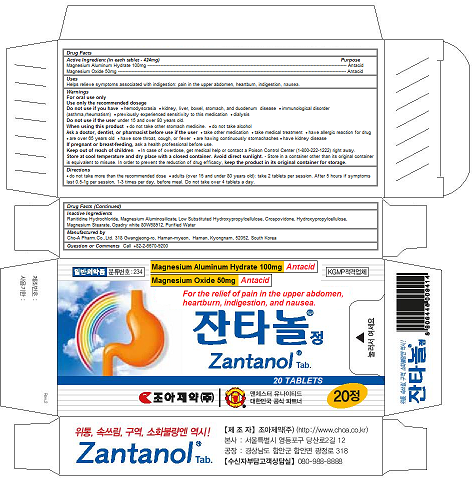
Label: ZANTANOL TAB.- magnesium oxide, magnesium aluminum hydrate tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 58354-101-01, 58354-101-02 - Packager: Cho-A Pharm.Co.,Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 28, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Keep out of reach of children
- Uses
-
Warnings
For oral use only
Use only the recommended dosage
Do not use if you have
• hemodyscrasia
• kidney, liver, bowel, stomach, and duodenum disease
• immunological disorder (asthma,rheumatism)
• previously experienced sensitivity to this medication
• dialysisDo not use if the user under 15 and over 80 years old.
When using this product
• do not take other stomach medicine.
• do not take alcoholAsk a doctor, dentist, or pharmacist before use if the user
• take other medication
• take medical treatment
• have allergic reaction for drug
• are over 65 years old
• have sore throat, cough, or fever
• are having continuously stomachaches
• have kidney diseaseIf pregnant or breast-feeding, ask a health professional before use.
Store at cool temperature and dry place with a closed container. Avoid direct sunlight. - Store in a container other than its original container is equivalent to misuse. In order to prevent the reduction of drug efficacy, keep the product in its original container for storage.
- Directions
- Inactive Ingredients
- Zantanol Tab.
-
INGREDIENTS AND APPEARANCE
ZANTANOL TAB.
magnesium oxide, magnesium aluminum hydrate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58354-101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM OXIDE 50 mg in 424 mg MAGALDRATE (UNII: 6V88E24N5T) (MAGALDRATE ANHYDROUS - UNII:0MFM55849I) MAGALDRATE 50 mg in 424 mg Inactive Ingredients Ingredient Name Strength ALMASILATE (UNII: OZQ8O62H53) RANITIDINE HYDROCHLORIDE (UNII: BK76465IHM) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE (8% HYDROXYPROPYL: 140000 MW) (UNII: U466TXA0AK) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) MAGNESIUM STEARATE (UNII: 70097M6I30) water (UNII: 059QF0KO0R) Product Characteristics Color white Score no score Shape capsule Size 13mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58354-101-02 10 in 1 PACKAGE 02/28/2017 1 NDC:58354-101-01 424 mg in 1 CAPSULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 02/28/2017 Labeler - Cho-A Pharm.Co.,Ltd. (688056831) Registrant - Cho-A Pharm.Co.,Ltd. (688056831) Establishment Name Address ID/FEI Business Operations Cho-A Pharm.Co.,Ltd. 688056831 manufacture(58354-101)