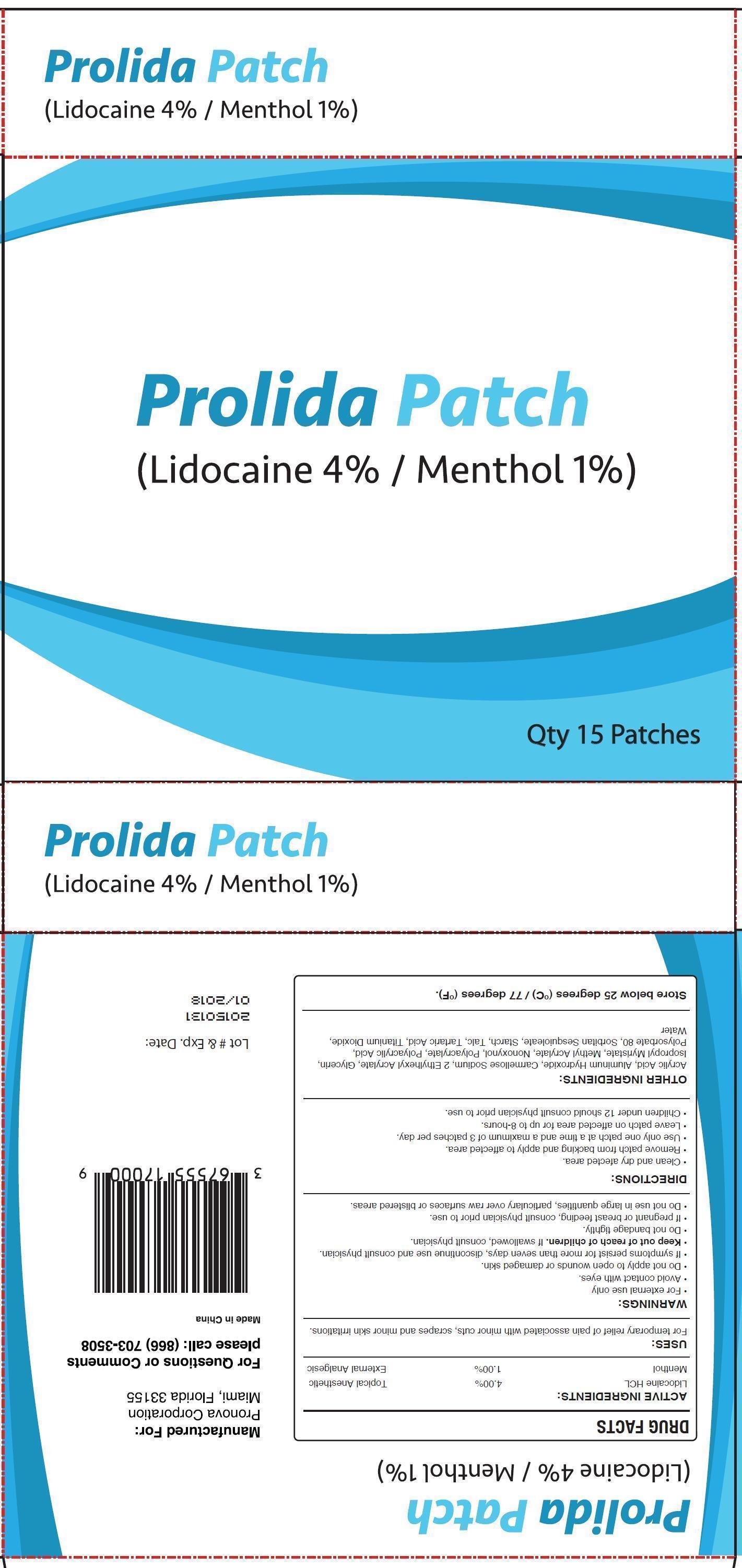
PROLIDA- lidocaine hydrochloride, menthol patch
Pronova Corp
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients:
Lidocaine HCL ..................... 4.00% ..................... Topical Anesthetic
Menthol ................................ 1.00% ................... External Analgesic
WARNINGS:
- For external use only
- Avoid contact with eyes
- Do not apply to open wounds or damaged skin
- If symptoms persist for more than seven days, discontinue use and consult physician.
- Do not bandage tightly
- Do not use in large quantities, particularly over raw serfaces or blistererd areas.
Directions:
- Clean and dry afected area.
- Remove patch from backing and apply to affected area.
- Use only one patch at a time and a maximum of 3 patches per day.
- Leave patch on affected area for up to 8-hours.
- Children under 12 should consult physician prior to use.
| PROLIDA
lidocaine hydrochloride, menthol patch |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Pronova Corp (111421496) |
Revised: 3/2017
Document Id: d7bacc73-aac4-40f4-98d7-35c683f32dc1
Set id: 9adf4ecb-6999-4504-ad28-7235cd2fbbdf
Version: 5
Effective Time: 20170302
Pronova Corp