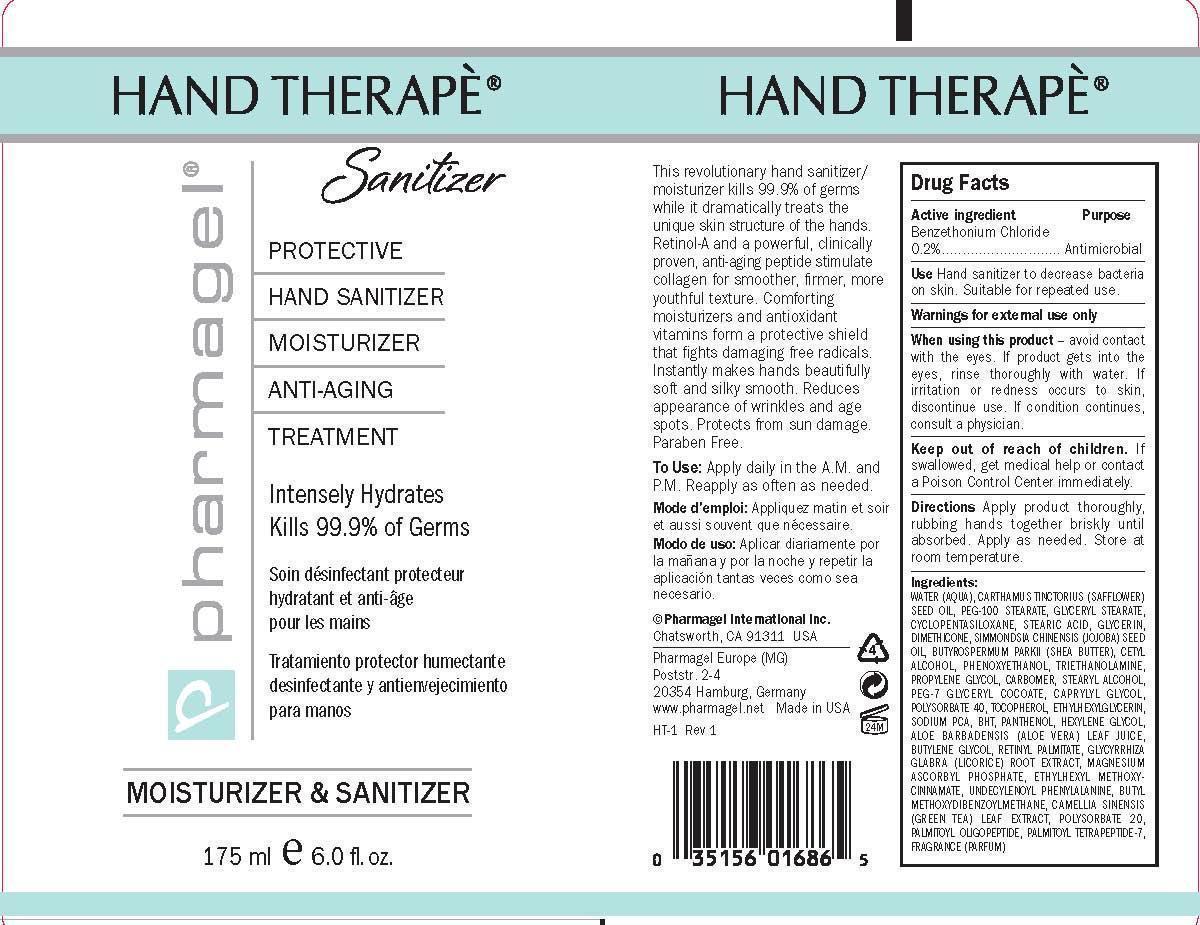
Label: HAND THERAPE SANITIZER- benzethonium chloride gel
- NDC Code(s): 67879-201-13, 67879-201-16, 67879-201-26
- Packager: PHARMAGEL INTERNATIONAL INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- PURPOSE
- USES
- WARNINGS
- When using this product:
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
-
INACTIVE INGREDIENTS:
WATER (AQUA), CARTHAMUS TINCTORIUS (SAFFLOWER) SEED OIL, PEG-100 STEARATE, GLYCERYL STEARATE, CYCLOPENTASILOXANE, STEARIC ACID, GLYCERIN, DIMETHICONE, SIMMONDSIA CHINENSIS (JOJOBA) SEED OIL, BUTYROSPERMUM PARKII (SHEA BUTTER), CETYL ALCOHOL, PHENOXYETHANOL, TRIETHANOLAMINE, PROPYLENE GLYCOL, CARBOMER, STEARYL ALCOHOL, PEG-7 GLYCERYL COCOATE, CAPRYLYL GLYCOL, POLYSORBATE 40, TOCOPHEROL, ETHYLHEXYLGLYCERIN, SODIUM PCA, BHT, PANTHENOL, HEXYLENE GLYCOL, ALOE BARBADENSIS (ALOE VERA) LEAF JUICE, BUTYLENE GLYCOL, RETINYL PALMITATE, GLYCYRRHIZA GLABRA (LICORICE) ROOT EXTRACT, MAGNESIUM ASCORBYL PHOSPHATE, ETHYLHEXYL METHOXYCINNAMATE, UNDECYLENOYL PHENYLALANINE, BUTYL METHOXYDIBENZOYLMETHANE, CAMELLIA SINENSIS(GREEN TEA) LEAF EXTRACT, POLYSORBATE 20, PALMITOYL OLIGOPEPTIDE, PALMITOYL TETRAPEPTIDE-7, FRAGRANCE (PARFUM)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HAND THERAPE SANITIZER
benzethonium chloride gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67879-201 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZETHONIUM CHLORIDE (UNII: PH41D05744) (BENZETHONIUM - UNII:1VU15B70BP) BENZETHONIUM CHLORIDE 0.2 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SAFFLOWER OIL (UNII: 65UEH262IS) PEG-100 STEARATE (UNII: YD01N1999R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) JOJOBA OIL (UNII: 724GKU717M) SHEANUT OIL (UNII: O88E196QRF) CETYL ALCOHOL (UNII: 936JST6JCN) PHENOXYETHANOL (UNII: HIE492ZZ3T) TROLAMINE (UNII: 9O3K93S3TK) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) PEG-7 GLYCERYL COCOATE (UNII: VNX7251543) CAPRYLYL GLYCOL (UNII: 00YIU5438U) POLYSORBATE 40 (UNII: STI11B5A2X) TOCOPHEROL (UNII: R0ZB2556P8) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PANTHENOL (UNII: WV9CM0O67Z) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALOE VERA LEAF (UNII: ZY81Z83H0X) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) OCTINOXATE (UNII: 4Y5P7MUD51) UNDECYLENOYL GLYCINE (UNII: 4D20464K2J) AVOBENZONE (UNII: G63QQF2NOX) GREEN TEA LEAF (UNII: W2ZU1RY8B0) POLYSORBATE 20 (UNII: 7T1F30V5YH) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67879-201-13 88 mL in 1 TUBE; Type 0: Not a Combination Product 06/18/2015 2 NDC:67879-201-16 175 mL in 1 PACKAGE; Type 0: Not a Combination Product 06/18/2015 3 NDC:67879-201-26 500 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/18/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 06/18/2015 Labeler - PHARMAGEL INTERNATIONAL INC (603215182)