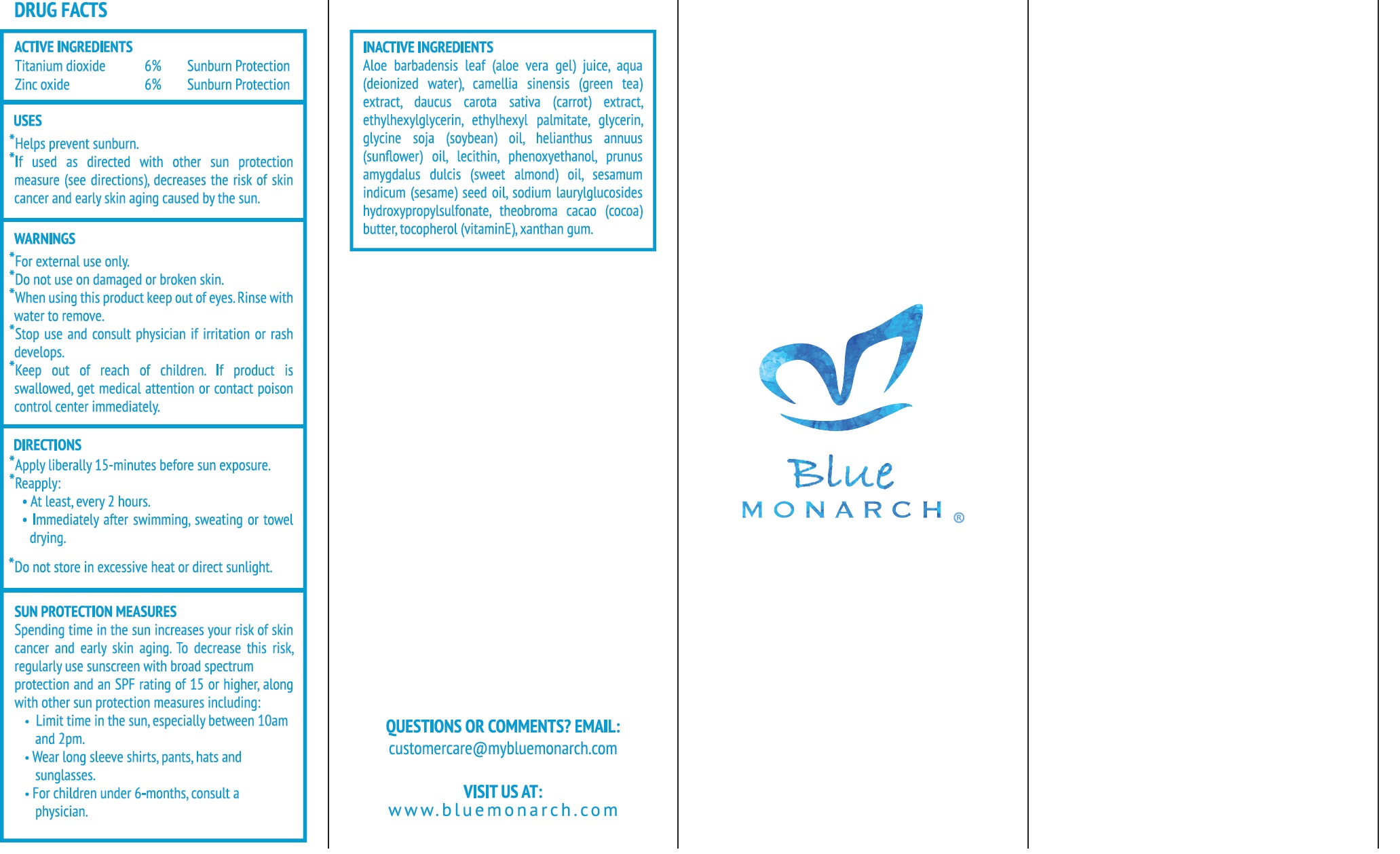
ACTIVE INGREDIENTS
Titanium dioxide 6%
Zinc oxide 6%
Purpose
Sunburn Protection
USES
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see directions), decreases the risk of skin cancer and early skin aging caused by the sun.
WARNINGS
For external use only.
Do not use
on damaged or broken skin.
When using this product
keep out of eyes. Rinse with water to remove.
Stop use and consult physician if
irritation or rash develops.
Keep out of reach of children.
If product is swallowed, get medical attention or contact poison control center immediately.
DIRECTIONS
- Apply liberally 15-minutes before sun exposure.
- Reapply:
- At least, every 2 hours.
- Immediately after swimming, sweating or towel drying.
- Do not store in excessive heat or direct sunlight.
SUN PROTECTION MEASURES
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use sunscreen with broad spectrum protection and an SPF rating of 15 or higher, along with other sun protection measures including:
- Limit time in the sun, especially between 10am and 2pm
- Wear long sleeve shirts, pants, hats and sunglasses.
- For children under 6-months, consult a physician.
INACTIVE INGREDIENTS
Aloe barbadensis leaf (aloe vera gel) juice, aqua (deionized water), camellia sinensis (green tea) extract, daucus carota sativa (carrot) extract, ethylhexylglycerin, ethylhexyl palmitate, glycerin, glycine soja (soybean) oil, helianthus annuus (sunflower) oil, lecithin, phenoxyethanol, prunus amygdalus dulcis (sweet almond) oil, sesamum indicum (sesame) seed oil, sodium laurylglucosides hydroxypropylsulfonate, theobroma cacao (cocoa) butter, tocopherol (vitaminE), xanthan gum.
Package Labeling