DIPHENHYDRAMINE HCL- diphenhydramine hydrochloride syrup
Delsam Pharma Llc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
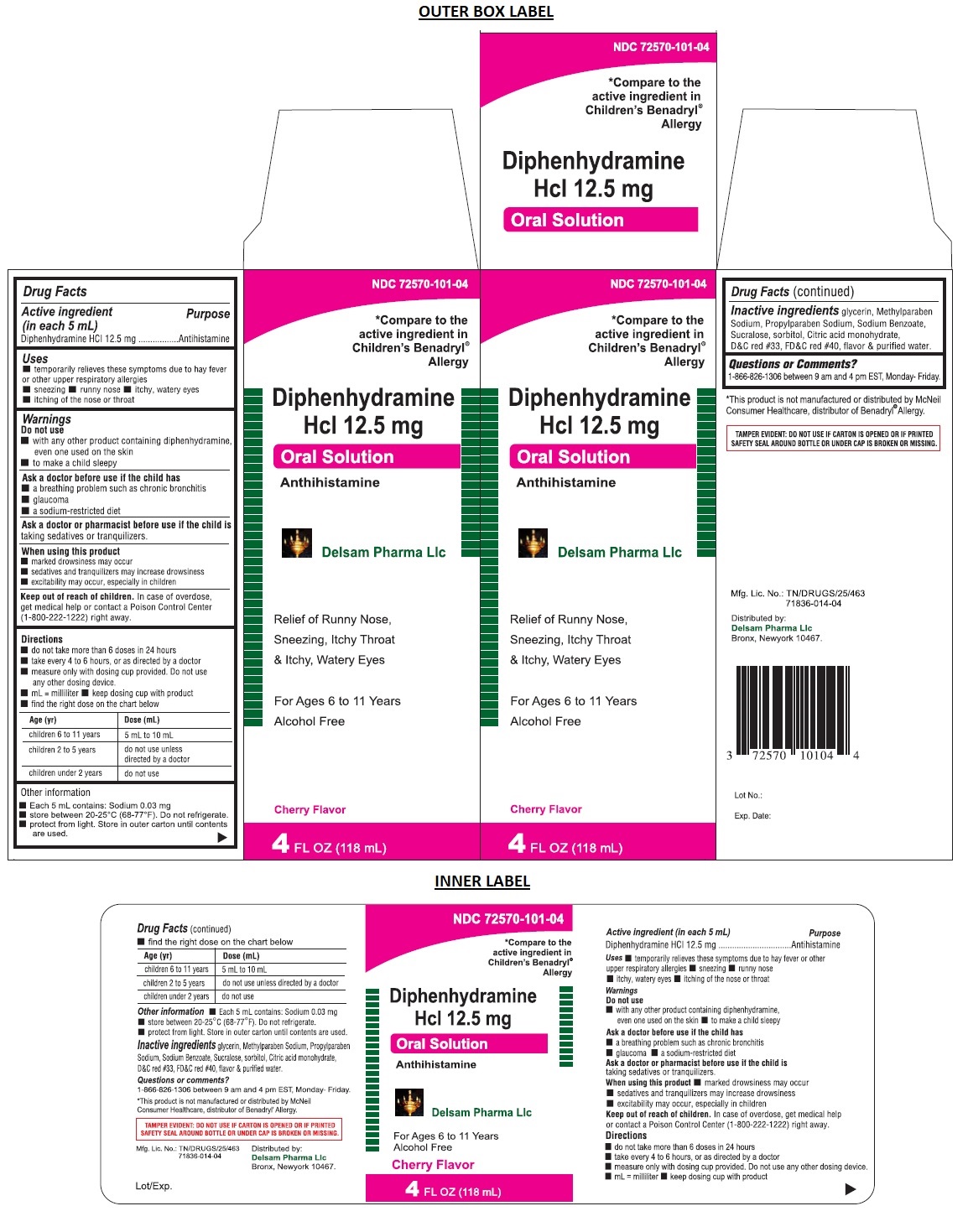
Diphenhydramine Hcl 12.5 mg Oral Solution
Uses
• temporarily relieves these symptoms due to hay fever or other upper respiratory allergies
• sneezing • runny nose • itchy, watery eyes • itching of the nose or throat
Warnings
Do not use
• with any other product containing diphenhydramine, even one used on the skin
• to make a child sleepy
Ask a doctor before use if the child has
• a breathing problem such as chronic bronchitis
• glaucoma
• a sodium-restricted diet
Ask a doctor or pharmacist before use if the child is taking sedatives or tranquilizers.
When using this product
• marked drowsiness may occur
• sedatives and tranquilizers may increase drowsiness
• excitability may occur, especially in children
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
• do not take more than 6 doses in 24 hours
• take every 4 to 6 hours, or as directed by a doctor
• measure only with dosing cup provided. Do not use any other dosing device.
• mL=milliliter • keep dosing cup with product
• find the right dose on the chart below
| Age (yr) | Dose (mL) |
| children 6 to 11 years | 5 mL to 10 mL |
| children 2 to 5 years | do not use unless directed by a doctor |
| children under 2 years | do not use |
Inactive ingredients
glycerin, Methylparaben Sodium, Propylparaben Sodium, Sodium Benzoate, Sucralose, sorbitol, Citric acid monohydrate, D&C red #33, FD&C red #40, flavor & purified water.
Other information
• Each 5 mL contains: Sodium 0.03 mg
• store between 20-25°C (68-77°F). Do not refrigerate.
• protect from light. Store in outer carton until contents are used.
*Compare to the active ingredient in Children's Benadryl® Allergy
Relief of Runny Nose, Sneezing, Itchy Throat & Itchy, Watery Eyes
For Ages 6 to 11 Years
Alcohol Free
Cherry Flavor
* This product is not manufactured or distributed by McNeil Consumer Healthcare, distributor of Benadryl® Allergy.
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF PRINTED SAFETY SEAL AROUND BOTTLE OR UNDER CAP IS BROKEN OR MISSING.
Distributed by:
Delsam Pharma Llc
Bronx, Newyork 10467.
| DIPHENHYDRAMINE HCL
diphenhydramine hydrochloride syrup |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Delsam Pharma Llc (081369679) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CENTRAL DRUGS & PHARMACEUTICALS | 677141426 | manufacture(72570-101) | |