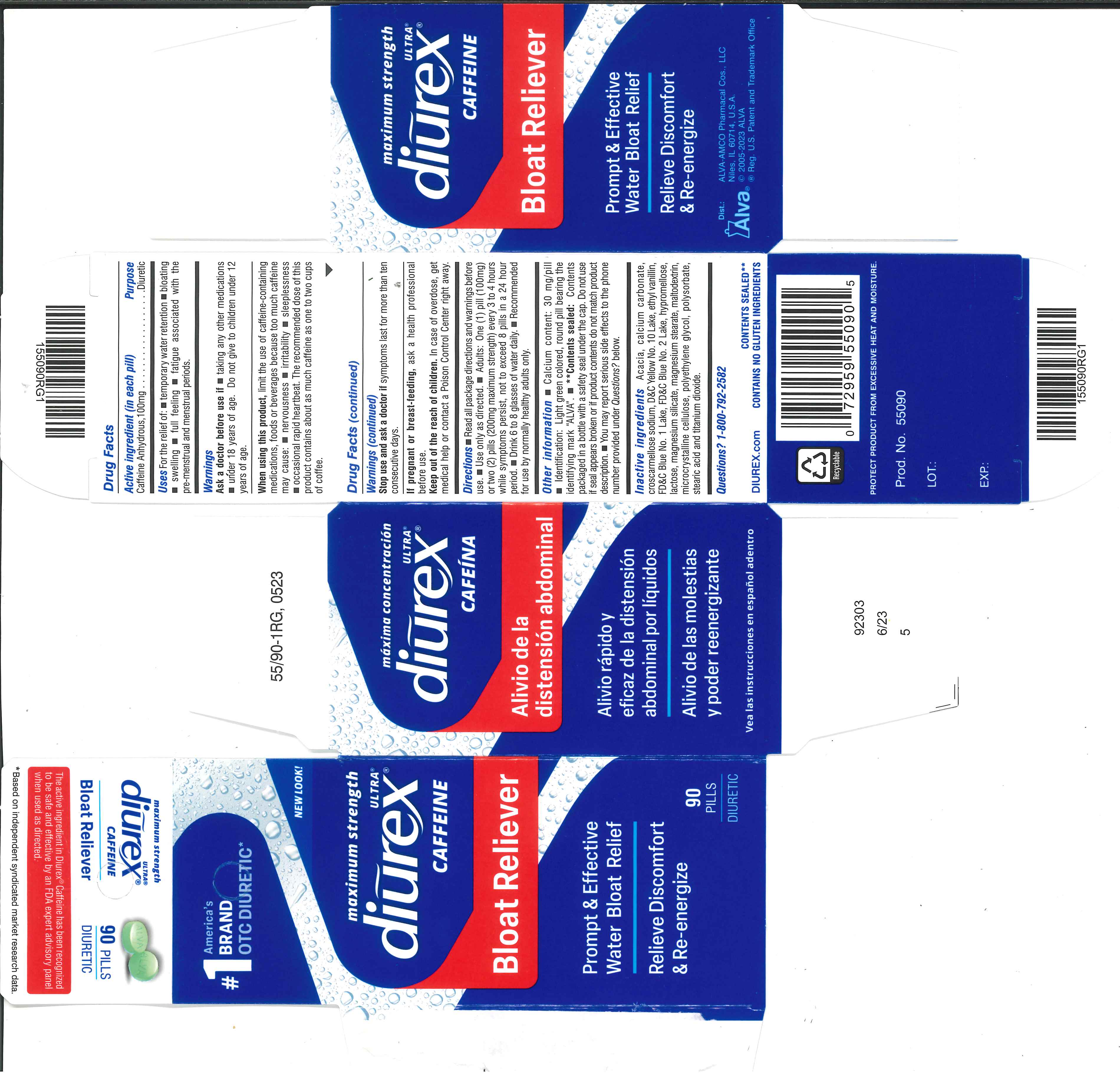
Label: DIUREX ULTRA- caffeine tablet, film coated
- NDC Code(s): 52389-155-80, 52389-155-90
- Packager: Alva-Amco Pharmacal Companies, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each pill)
- Purpose
- Uses
- Warnings
- Ask a doctor before use if
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- Read all package directions and warnings before use.
- Use only as directed.
- Adults: One (1) pill (100 mg) or two (2) pills (200 mg maximum strength) every 3 to 4 hours wile symptoms persist, not to exceed 8 pills in a 24 hour period.
- Drink 6 to 8 glasses of water daily.
- Recommended for use by normally healthy adults only.
-
Other information
- Calcium content: 30 mg/pill
- Identification: Light green colored, round pill bearing the identifying mark "ALVA".
- **Contents sealed: Contents packaged in a bottle with a safety seal under the cap. Do not use if seal appears broken or if product contents do not match product description.
- You may report serious side effects to the phone number provided under Questions? below.
-
Inactive ingredients
Acacia, calcium carbonate, croscarmellose sodium, D&C Yellow No. 10 Lake, ethyl vanillin, FD&C Blue No. 1 Lake, FD&C Blue No. 2 Lake, hypromellose, lactose, magnesium silicate, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, polysorbate, stearic acid, titanium dioxide.
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DIUREX ULTRA
caffeine tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52389-155 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 100 mg Inactive Ingredients Ingredient Name Strength D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) HYPROMELLOSES (UNII: 3NXW29V3WO) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) ACACIA (UNII: 5C5403N26O) CALCIUM CARBONATE (UNII: H0G9379FGK) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) ETHYL VANILLIN (UNII: YC9ST449YJ) Product Characteristics Color green (light green) Score no score Shape ROUND Size 10mm Flavor Imprint Code ALVA Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52389-155-80 80 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/01/2005 2 NDC:52389-155-90 1 in 1 CARTON 09/01/2005 2 90 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M027 09/01/2005 Labeler - Alva-Amco Pharmacal Companies, Inc. (042074856)