NOVOLIN 70/30
-
insulin human injection, suspension
Novo Nordisk
----------
PATIENT PACKAGE INSERT
Patient Information for Novolin® 70/30
NOVOLIN® 70/30 (NO-voe-lin)
70% NPH, Human Insulin Isophane Suspension and
30% Regular, Human Insulin Injection
(recombinant DNA origin) 100 units/mL
Important:
Know your insulin. Do not change the type of insulin you use unless told to do so by your healthcare provider. The amount of insulin you take as well as the best time for you to take your insulin may need to change if you take a different type of insulin.
Make sure that you know the type and strength of insulin that is prescribed for you.
Read the Patient Information leaflet that comes with Novolin 70/30 before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your diabetes or your treatment. Make sure you know how to manage your diabetes. Ask your healthcare provider if you have any questions about managing your diabetes.
What is Novolin 70/30?
Novolin 70/30 is a man-made insulin (recombinant DNA origin) which is a mixture of 70% NPH, Human Insulin Isophane Suspension and 30% Regular, Human Insulin Injection that is structurally identical to the insulin produced by the human pancreas that is used to control high blood sugar in patients with diabetes mellitus.
Who should not use Novolin 70/30?
Do not take Novolin 70/30 if:
- Your blood sugar is too low (hypoglycemia).
- You are allergic to anything in Novolin 70/30. See the end of this leaflet for a complete list of ingredients in Novolin 70/30. Check with your healthcare provider if you are not sure.
Tell your healthcare provider:
- about all of your medical conditions. Medical conditions can affect your insulin needs and your dose of Novolin 70/30.
- if you are pregnant or breastfeeding. You and your healthcare provider should talk about the best way to manage your diabetes while you are pregnant or breastfeeding. Novolin 70/30 has not been studied in pregnant or nursing women.
- about all of the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. Many medicines can affect your blood sugar levels and your insulin needs. Your Novolin 70/30 dose may need to change if you take other medicines.
Know the medicines you take. Keep a list of your medicines with you to show all your healthcare providers when you get a new medicine.
How should I take Novolin 70/30?
Only use Novolin 70/30 if it appears cloudy or milky. There may be air bubbles. This is normal. If the precipitate (the white deposit at the bottom of the vial) has become lumpy or granular in appearance or has formed a deposit of solid particles on the wall of the vial, do not use it, and call Novo Nordisk at 1-800-727-6500. This insulin should not be used if the liquid in the vial remains clear after the vial has been gently rotated.
Novolin 70/30 comes in:
- 10 mL vials (small bottles) for use with syringe
Read the instructions for use that come with your Novolin 70/30 product. Talk to your healthcare provider if you have any questions. Your healthcare provider should show you how to inject Novolin 70/30 before you start taking it. Follow your healthcare provider’s instructions to make changes to your insulin dose.
- Take Novolin 70/30 exactly as prescribed.
- Novolin 70/30 is an intermediate-acting insulin. The effects of Novolin 70/30 start working ½ hour after injection.
- The greatest blood sugar lowering effect is between 2 and 12 hours after the injection. This blood sugar lowering may last up to 24 hours.
- While using Novolin 70/30, any change of insulin should be made cautiously and only under medical supervision. Doses of oral anti-diabetic medicines may also need to change, if your insulin is changed.
- Do not mix Novolin 70/30 with any insulins.
- Inject Novolin 70/30 into the skin of your stomach area, upper arms, buttocks or upper legs. Novolin 70/30 may affect your blood sugar levels sooner if you inject it into the skin of your stomach area. Never inject Novolin 70/30 into a vein or into a muscle.
- Change (rotate) your injection site within the chosen area (for example, stomach or upper arm) with each dose. Do not inject into the same spot for each injection.
- If you take too much Novolin 70/30, your blood sugar may fall low (hypoglycemia). You can treat mild low blood sugar (hypoglycemia) by drinking or eating something sugary right away (fruit juice, sugar candies, or glucose tablets). It is important to treat low blood sugar (hypoglycemia) right away because it could get worse and you could pass out (become unconscious). If you pass out, you will need help from another person or emergency medical services right away, and will need treatment with a glucagon injection or treatment at a hospital. See “What are the possible side effects of Novolin 70/30?” for more information on low blood sugar (hypoglycemia).
- If you forget to take your dose of Novolin 70/30, your blood sugar may go too high (hyperglycemia). If high blood sugar (hyperglycemia) is not treated it can lead to diabetic ketoacidosis, which can lead to serious problems, like loss of consciousness (passing out), coma or even death. Follow your healthcare provider’s instructions for treating high blood sugar (hyperglycemia), and talk to your healthcare provider if high blood sugar is a problem for you. Severe or continuing high blood sugar (hyperglycemia) requires prompt evaluation and treatment by your healthcare provider. Know your symptoms of high blood sugar (hyperglycemia) and diabetic ketoacidosis which may include:
|
|
|
|
|
|
|
|
- Check your blood sugar levels. Ask your healthcare provider how often you should check your blood sugar levels for hypoglycemia (too low blood sugar) and hyperglycemia (too high blood sugar).
Your insulin dosage may need to change because of:
|
|
|
|
|
|
See the end of this patient information for instructions about preparing and giving the injection.
What should I avoid while using Novolin 70/30?
- Alcohol. Alcohol, including beer and wine, may affect your blood sugar when you take Novolin 70/30.
-
Driving and operating machinery. You may have difficulty concentrating or reacting if you have low blood sugar (hypoglycemia). Be careful when you drive a car or operate machinery. Ask your healthcare provider if it is alright to drive if you often have:
- low blood sugar
- decreased or no warning signs of low blood sugar
What are the possible side effects of Novolin 70/30?
- Low blood sugar (hypoglycemia). Symptoms of hypoglycemia (low blood sugar) may include:
|
|
|
|
|
|
|
|
|
|
|
Severe low blood sugar (hypoglycemia) can cause unconsciousness (passing out), seizures, and death. Know your symptoms of low blood sugar. Follow your healthcare provider’s instructions for treating low blood sugar. Talk to your healthcare provider if low blood sugar is a problem for you.
- Serious allergic reaction (whole body reaction). Get medical help right away if you develop a rash over your whole body, have trouble breathing, a fast heartbeat, or sweating.
- Reactions at the injection site (local allergic reaction). You may get redness, swelling, and itching at the injection site. If you keep having skin reactions, or they are serious, talk to your healthcare provider. You may need to stop using Novolin 70/30 and use a different insulin. Do not inject insulin into skin that is red, swollen, or itchy.
- Skin thickens or pits at the injection site (lipodystrophy). Change (rotate) where you inject your insulin to help prevent these skin changes from happening. Do not inject insulin into this type of skin.
- Swelling of your hands and feet
- Vision changes
- Low potassium in your blood (hypokalemia)
These are not all of the possible side effects from Novolin 70/30. Ask your healthcare provider or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Novolin 70/30?
All Unopened Novolin 70/30:
- Keep all unopened Novolin 70/30 in the refrigerator between 36° to 46°F (2° to 8°C).
- Do not freeze. Do not use Novolin 70/30 if it has been frozen.
- If refrigeration is not possible, the unopened vial may be kept at room temperature for up to 6 weeks (42 days), as long as it is kept at or below 77°F (25°C).
- Keep unopened Novolin 70/30 in the carton to protect from light.
Novolin 70/30 in use:
Vials
- Keep at room temperature below 77°F (25°C) for up to 6 weeks (42 days).
- Keep vials away from direct heat or light.
- Throw away an opened vial after 6 weeks (42 days) of use, even if there is insulin left in the vial.
- Unopened vials can be used until the expiration date on the Novolin 70/30 label, if the medicine has been stored in a refrigerator.
General advice about Novolin 70/30
Novolin 70/30 is used for the treatment of diabetes only. Medicines are sometimes prescribed for conditions that are not mentioned in the patient leaflet. Do not use Novolin 70/30 for a condition for which it was not prescribed. Do not give Novolin 70/30 to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about Novolin 70/30. If you would like more information about Novolin 70/30 or diabetes, talk with your healthcare provider. For more information, call 1-800-727-6500 or visit www.novonordisk-us.com.
Helpful information for people with diabetes is published by the American Diabetes Association, 1701 N Beauregard Street, Alexandria, VA 22311 and on www.diabetes.org.
Novolin 70/30 ingredients include:
- 70% NPH, Human Insulin Isophane Suspension and 30% Regular, Human Insulin Injection (recombinant DNA origin)
Novolin 70/30 ingredients include:
|
|
|
|
|
|
|
|
|
All Novolin 70/30 vials are latex-free.
ReliOn®
Date of issue: May 14, 2010
Version: 5
Novolin® and Novo Nordisk® are trademarks of Novo Nordisk A/S.
ReliOn® is a trademark owned by Access LLC
ReliOn® is licensed by Novo Nordisk Inc.
© 2005-2010 Novo Nordisk A/S
Manufactured by:
Novo Nordisk A/S
DK-2880 Bagsvaerd, Denmark
For information about Novolin 70/30 contact:
Novo Nordisk Inc.
100 College Road West
Princeton, New Jersey 08540
Patient Instructions for Use
Novolin® 70/30 10 mL vial (100 Units/mL, U-100)
Before starting, gather all of the supplies that you will need to use for preparing and giving your insulin injection.
Never re-use syringes and needles.
How should I use the Novolin 70/30 vial?
- Check to make sure that you have the correct type of insulin.
- Look at the vial and the insulin. The insulin should be a cloudy or milky suspension. The tamper-resistant cap should be in place before the first use. If the cap had been removed before your first use of the vial, or if the precipitate (the white deposit at the bottom of the vial) has become lumpy or granular in appearance or has formed a deposit of solid particles on the wall of the vial, do not use it, and call Novo Nordisk at 1-800-727-6500.
- Wash your hands with soap and water. If you clean your injection site with an alcohol swab, let the injection site dry before you inject. Talk with your healthcare provider about how to rotate injection sites and how to give an injection.
- If you are using a new vial, pull off the tamper-resistant cap. Wipe the rubber stopper with an alcohol swab.
- Roll the vial gently 10 times in your hands to mix it. This procedure should be carried out with the vial in a horizontal position. The rolling procedure must be repeated until the suspension appears uniformly white and cloudy. Shaking right before the dose is drawn into the syringe may cause bubbles or froth, which could cause you to draw up the wrong dose of insulin.
- Pull back the plunger on the syringe until the black tip reaches the marking for the number of units you will inject.
- Push the needle through the rubber stopper of the vial, and push the plunger all the way in to force air into the vial.
- Turn the vial and syringe upside down and slowly pull the plunger back to a few units beyond the correct dose.
- If there are any air bubbles, tap the syringe gently with your finger to raise the air bubbles to the top. Then slowly push the plunger to the marking for your correct dose. This process should move any air bubbles present in the syringe back into the vial.
- Check to make sure you have the right dose of Novolin 70/30 in the syringe.
- Pull the syringe with needle out of the vial’s rubber stopper.
- Your doctor should tell you if you need to pinch the skin before inserting the needle. This can vary from patient to patient so it is important to ask your doctor if you did not receive instructions on pinching the skin. Insert the needle into the skin. Press the plunger of the syringe to inject the insulin. When you are finished injecting the insulin, pull the needle out of your skin. You may see a drop of Novolin 70/30 at the needle tip. This is normal and has no effect on the dose you just received. If you see blood after you take the needle out of your skin, press the injection site lightly with a piece of gauze or an alcohol wipe. Do not rub the area.
- After your injection, do not recap the needle. Place used syringes, needles and used insulin vials in a disposable puncture-resistant sharps container, or some type of hard plastic or metal container with a screw on cap such as a detergent bottle or coffee can.
- Ask your healthcare provider about the right way to throw away used syringes and needles. There may be state or local laws about the right way to throw away used syringes and needles. Do not throw away used needles and syringes in household trash or recycle.
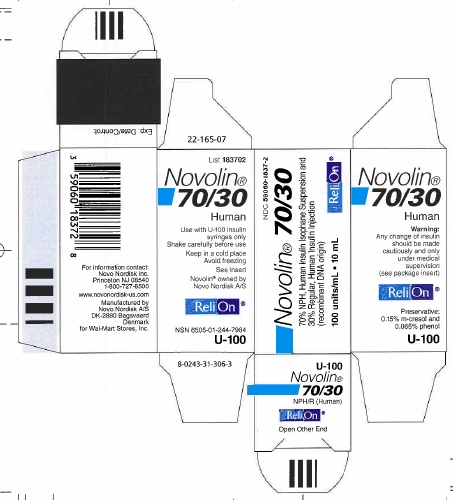
Principal Display Panel
NDC 59060-1837-2
Novolin® 70/30
70% NPH, Human Insulin Isophane Suspension and
30% Regular, Human Insulin Injection
(recombinant DNA origin)
100 units/mL
ReliOn®
| NOVOLIN
70/30
human insulin injection, suspension |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA019991 | 06/15/2000 | 08/31/2012 |
| Labeler - Novo Nordisk (012177531) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Novo Nordisk Pharmaceuticals Industries Inc. | 622920320 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Novo Nordisk A/S | 312296002 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Novo Nordisk A/S | 305156788 | API MANUFACTURE | |
Revised: 02/2012 Novo Nordisk