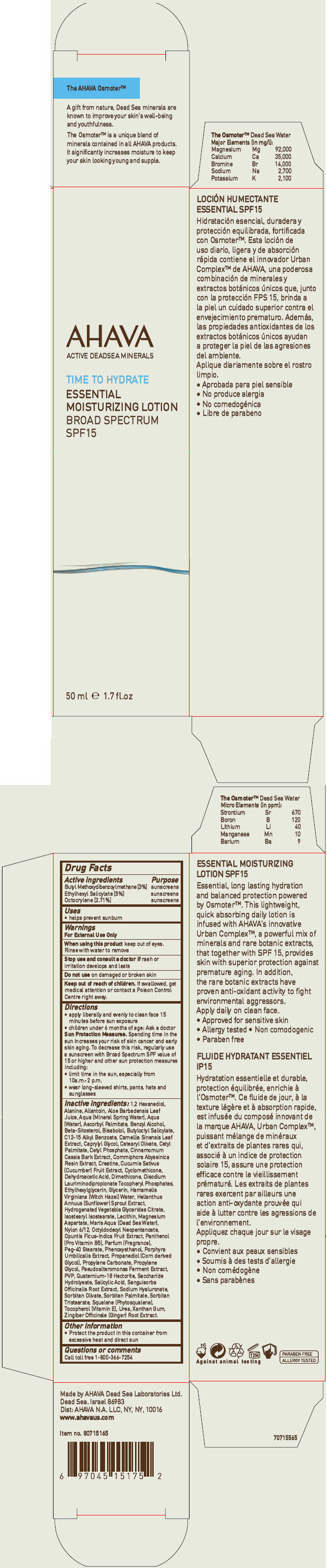
AHAVA ACTIVE DEADSEA MINERALS TIME TO HYDRATE ESSENTIAL MOISTURIZING BROAD SPECTRUM SPF15- avobenzone, octisalate, and octocrylene lotion
AHAVA Dead Sea Laboratories Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
AHAVA ACTIVE DEADSEA MINERALS
TIME TO HYDRATE ESSENTIAL MOISTURIZING BROAD SPECTRUM SPF15
Directions
- apply liberally and evenly to clean face 15 minutes before sun exposure
- children under 6 months of age: Ask a doctor
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10a.m.-2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
Inactive ingredients
1,2 Hexanediol, Alanine, Allantoin, Aloe Barbadensis Leaf Juice, Aqua (Mineral Spring Water), Aqua (Water), Ascorbyl Palmitate, Benzyl Alcohol, Beta-Sitosterol, Bisabolol, Butyloctyl Salicylate, C12-15 Alkyl Benzoate, Camellia Sinensis Leaf Extract, Caprylyl Glycol, Cetearyl Olivate, Cetyl Palmitate, Cetyl Phosphate, Cinnamomum Cassia Bark Extract, Commiphora Abyssinica Resin Extract, Creatine, Cucumis Sativus (Cucumber) Fruit Extract, Cyclomethicone, Dehydroacetic Acid, Dimethicone, Disodium Lauriminodipropionate Tocopheryl Phosphates, Ethylhexylglycerin, Glycerin, Hamamelis Virginiana (Witch Hazel) Water, Helianthus Annuus (Sunflower) Sprout Extract, Hydrogenated Vegetable Glycerides Citrate, Isostearyl Isostearate, Lecithin, Magnesium Aspartate, Maris Aqua (Dead Sea Water), Nylon 6/12, Octyldodecyl Neopentanoate, Opuntia Ficus-Indica Fruit Extract, Panthenol (Pro Vitamin B5), Parfum (Fragrance), Peg-40 Stearate, Phenoxyethanol, Porphyra Umbilicalis Extract, Propanediol (Corn derived Glycol), Propylene Carbonate, Propylene Glycol, Pseudoalteromonas Ferment Extract, PVP, Quaternium-18 Hectorite, Saccharide Hydrolysate, Salicylic Acid, Sanguisorba Officinalis Root Extract, Sodium Hyaluronate, Sorbitan Olivate, Sorbitan Palmitate, Sorbitan Tristearate, Squalene (Phytosqualene), Tocopherol (Vitamin E), Urea, Xanthan Gum, Zingiber Officinale (Ginger) Root Extract.
| AHAVA ACTIVE DEADSEA MINERALS
TIME TO HYDRATE ESSENTIAL MOISTURIZING BROAD SPECTRUM SPF15
avobenzone, octisalate, and octocrylene lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - AHAVA Dead Sea Laboratories Ltd (600056907) |