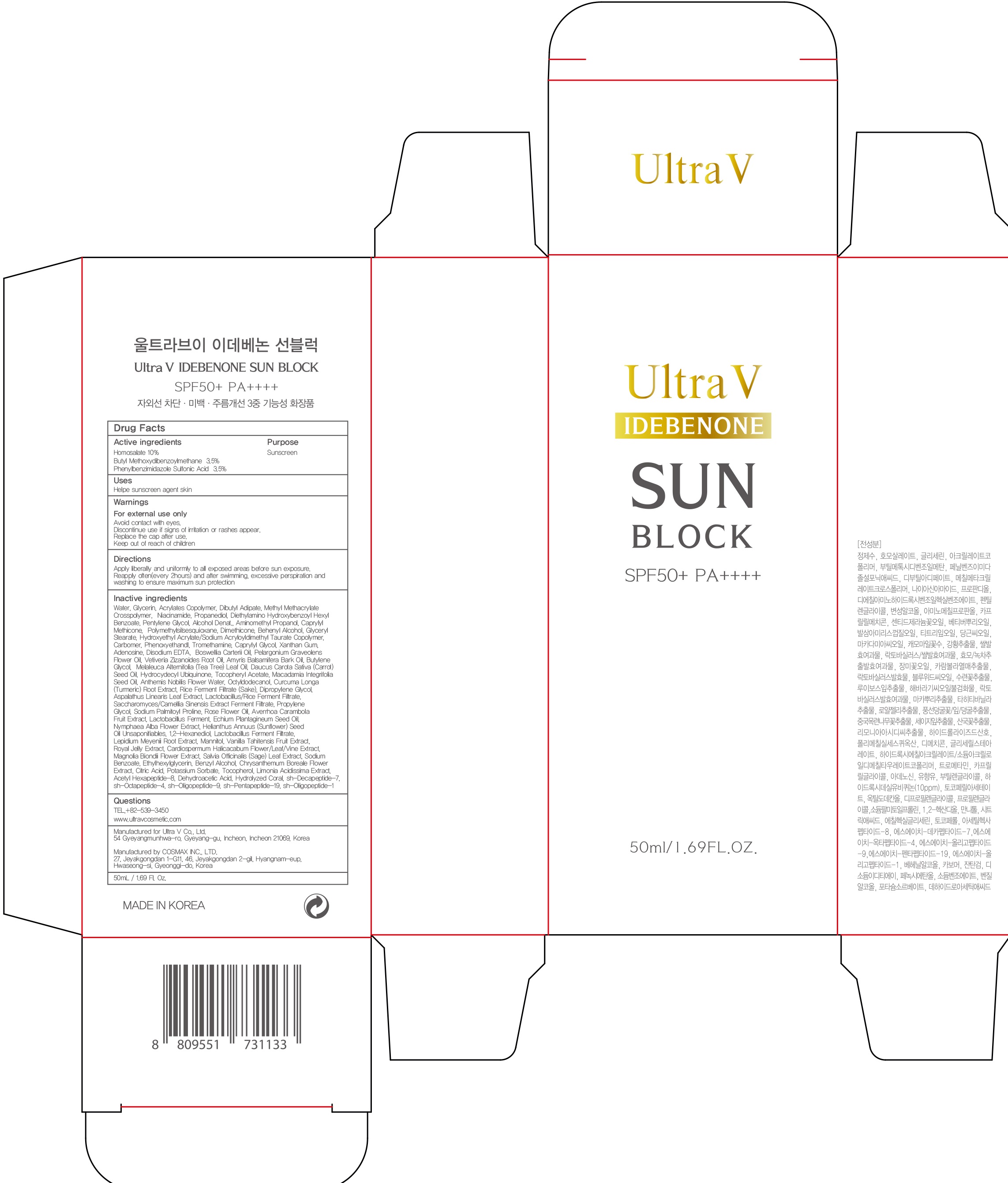
INACTIVE INGREDIENT
Inactive ingredients: Water, Glycerin, Acrylates Copolymer, Dibutyl Adipate, Methyl Methacrylate Crosspolymer, Niacinamide, Propanediol, Diethylamino Hydroxybenzoyl Hexyl Benzoate, Pentylene Glycol, Alcohol Denat., Aminomethyl Propanol, Caprylyl Methicone, Polymethylsilsesquioxane, Dimethicone, Behenyl Alcohol, Glyceryl Stearate, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Carbomer, Phenoxyethandl, Tromethamine, Caprylyl Glycol, Xanthan Gum, Adenosine, Disodium EDTA, Boswellia Carterii Oil, Pelargonium Graveolens Flower Oil, Vetiveria Zizanoides Root Oil, Amyris Balsamifera Bark Oil, Butylene Glycol, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Daucus Carota Sativa (Carrot) Seed Oil, Hydrocydecyl Ubiquinone, Tocopheryl Acetate, Macadamia Integrifolia Seed Oil, Anthemis Nobilis Flower Water, Octyldodecanol, Curcuma Longa (Turmeric) Root Extract, Rice Ferment Filtrate (Sake), Dipropylene Glycol, Aspalathus Linearis Leaf Extract, Lactobacillus/Rice Ferment Filtrate, Saccharomyces/Camellia Sinensis Extract Ferment Filtrate, Propylene Glycol, Sodium Palmitoyl Proline, Rose Flower Oil, Averrhoa Carambola Fruit Extract, Lactobacillus Ferment, Echium Plantagineum Seed Oil, Nymphaea Alba Flower Extract, Helianthus Annuus (Sunflower) Seed Oil Unsaponifiables, 1,2-Hexanediol, Lactobacillus Ferment Filtrate, Lepidium Meyenii Root Extract, Mannitol, Vanilla Tahitensis Fruit Extract, Royal Jelly Extract, Cardiospermum Halicacabum Flower/Leaf/Vine Extract, Magnolia Biondii Flower Extract, Salvia Officinalis (Sage) Leaf Extract, Sodium Benzoate, Ethylhexylglycerin, Benzyl Alcohol, Chrysanthemum Boreale Flower Extract, Citric Acid, Potassium Sorbate, Tocopherol, Limonia Acidissima Extract, , Acetyl Hexapeptide-8, Dehydroacetic Acid, Hydrolyzed Coral, sh-Decapeptide-7, sh-Octapeptide-4, sh-Oligopeptide-9, sh-Pentapeptide-19, sh-Oligopeptide-1