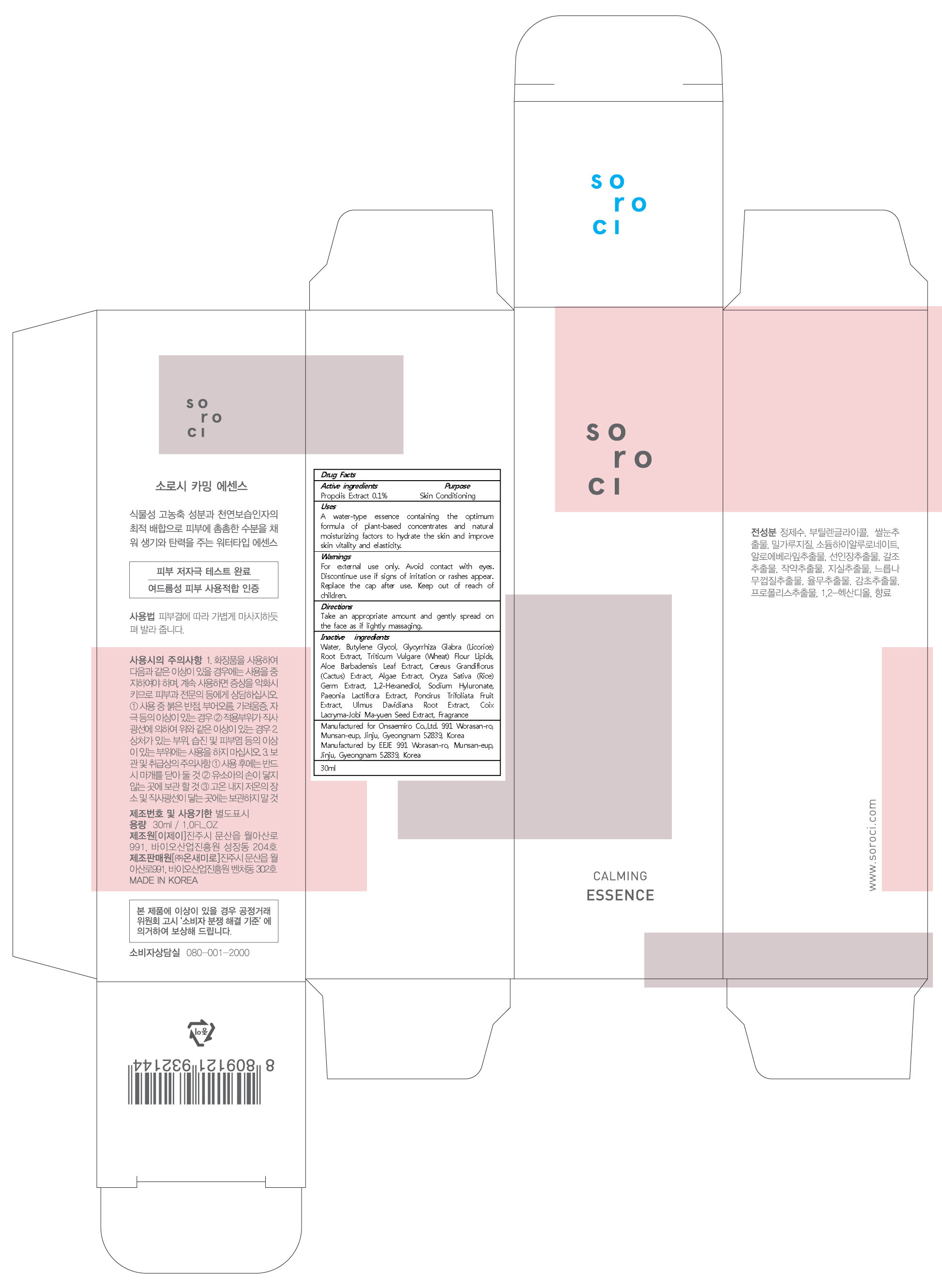
SOROCI CALMING ESSENCE- propolis liquid
Onsaemiro Co.,Ltd.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
INACTIVE INGREDIENT
Inactive ingredients:
Water, Butylene Glycol, Glycyrrhiza Glabra (Licorice) Root Extract, Triticum Vulgare (Wheat) Flour Lipids, Aloe Barbadensis Leaf Extract, Cereus Grandiflorus (Cactus) Extract, Algae Extract, Oryza Sativa (Rice) Germ Extract, 1,2-Hexanediol, Sodium Hyluronate, Paeonia Lactiflora Extract, Poncirus Trifoliata Fruit Extract, Ulmus Davidiana Root Extract, Coix Lacryma-Jobi Ma-yuen Seed Extract, Fragrance
WARNINGS
Warnings:
For external use only.
Avoid contact with eyes.
Discontinue use if signs of irritation or rashes appear.
Replace the cap after use.
Keep out of reach of children.
Uses
Uses:
A water-type essence containing the optimum formula of plant-based concentrates and natural moisturizing factors to hydrate the skin and improve skin vitality and elasticity.
| SOROCI CALMING ESSENCE
propolis liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Onsaemiro Co.,Ltd. (690366048) |
| Registrant - Onsaemiro Co.,Ltd. (690366048) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| EEJE | 688222932 | manufacture(71498-040) | |