BANOPHEN- diphenhydramine hcl capsule
Major Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Major 44-190-Delisted
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
- temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Directions
- take every 4 to 6 hours
- do not take more than 6 doses in 24 hours
| adults and children 12 years of age and over | 1 to 2 capsules |
| children 6 to under 12 years of age | 1 capsule |
| children under 6 years of age | do not use this product in children under 6 years of age |
Other information
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
- protect from moisture
Inactive ingredients
butylparaben, corn starch, D&C red #28, edible ink, FD&C blue #1, FD&C red #40, gelatin, lactose, magnesium stearate, methylparaben, polysorbate 80, propylparaben, silica gel
Principal Display Panel
MAJOR®
NDC 0904-2035-59
B A N O P H E N ™
Complete Allergy Medication
Diphenhydramine HCl 25 mg
ANTIHISTAMINE
For the temporary relief of the symptoms of:
• Upper Respiratory Allergies • Hay Fever
100 CAPSULES
Each Capsule Individually
Banded For Your Protection
*Compare to the
active ingredient in
BENADRYL®
*This product is not manufactured or distributed by
McNeil Consumer Healthcare, owner of the
registered trademark Benadryl®.
50844 REV0512J19012
Distributed by
MAJOR® PHARMACEUTICALS
31778 Enterprise Drive
Livonia, MI 48150 USA M-17 Rev. 09/12
Re-order No. 700695
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY
SEAL UNDER CAP IS BROKEN OR MISSING OR IF RED
BAND AROUND CAPSULE IS BROKEN OR MISSING
Major 44-190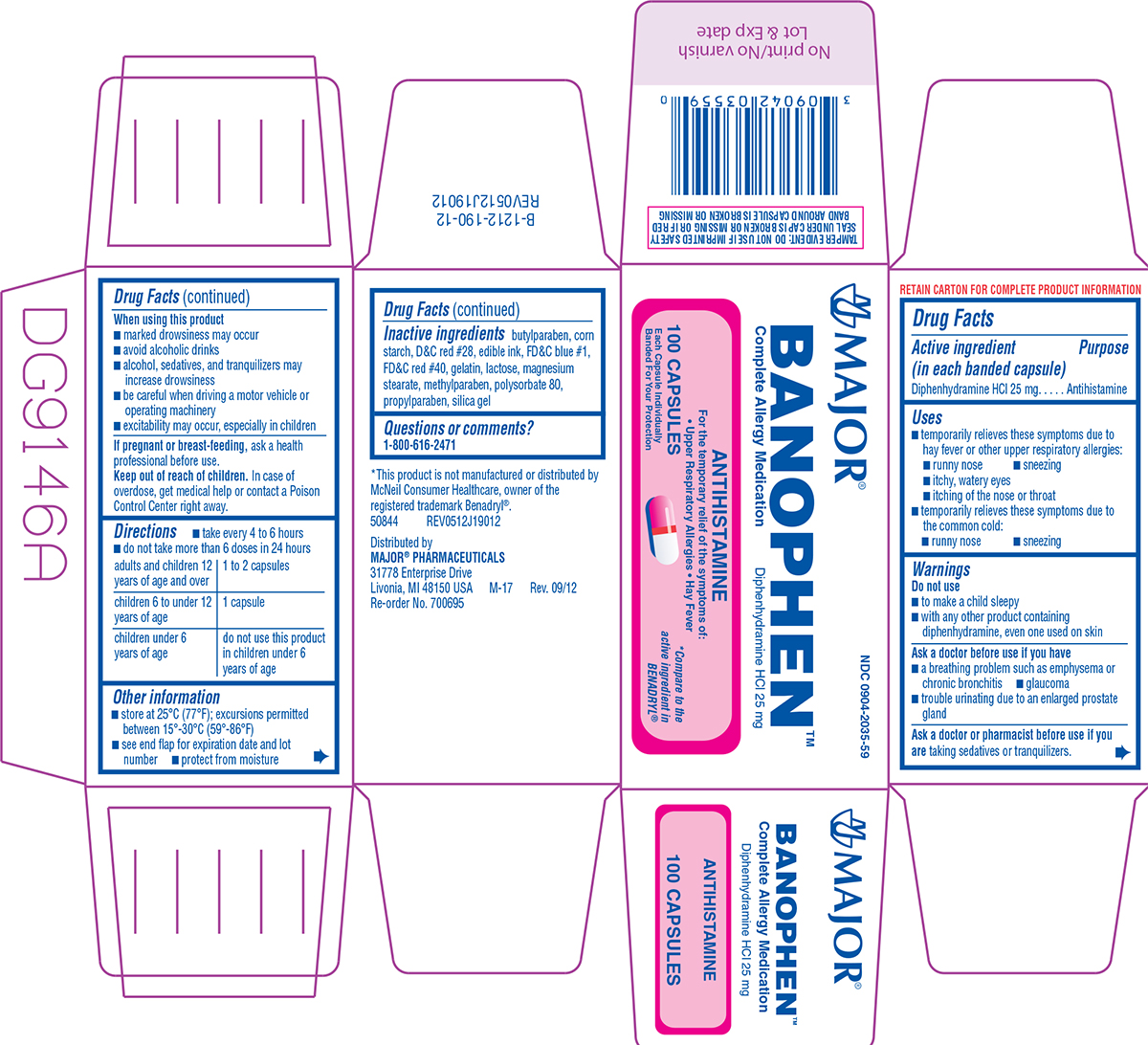
| BANOPHEN
diphenhydramine hcl capsule |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Major Pharmaceuticals (191427277) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | PACK(0904-2035) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | MANUFACTURE(0904-2035) , PACK(0904-2035) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | PACK(0904-2035) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | PACK(0904-2035) | |