Label: HUSH ANESTHETIC- lidocaine gel
- NDC Code(s): 49947-002-02, 49947-002-04
- Packager: HUSH Anesthetic
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- KEEP OUT OF REACH OF CHILDREN
- Uses
- Directions
- Warnings
- STOP USE
- DO NOT USE
- Other Information
-
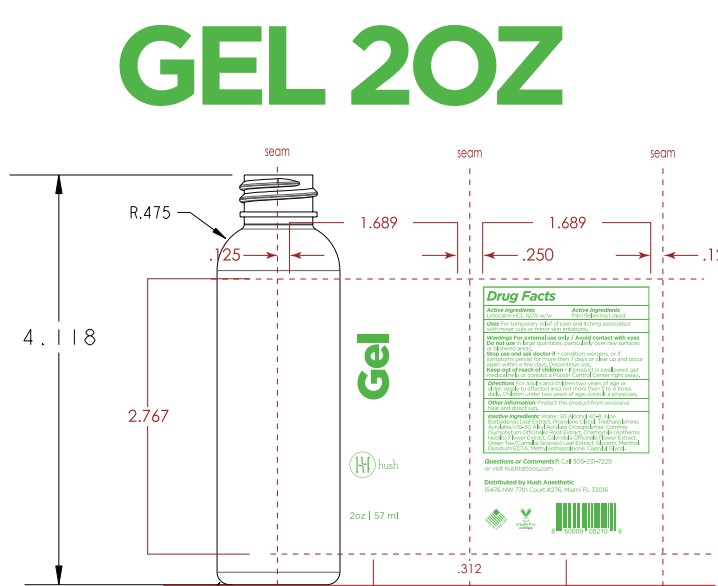
Inactive ingredients
Acrylates/c10-30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Extract, Aqua (Deionized Water), Calendula Officinale Extract, Caprylyl Glycol, Chamomile (Chamomile Recutita) Extract, Comfrey (Symphytum Officinale) Extract, Disodium EDTA, Glycerin, Green Tea (Camellia Sinensis) Extract, Menthol, Methylisothiazolinone, Propylene Glycol, SD Alcohol 40B, Triethanolamine
- Questions or Comments?
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HUSH ANESTHETIC
lidocaine gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49947-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 40 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) ALOE VERA LEAF (UNII: ZY81Z83H0X) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TROLAMINE (UNII: 9O3K93S3TK) GLYCERIN (UNII: PDC6A3C0OX) MENTHOL (UNII: L7T10EIP3A) CHAMAEMELUM NOBILE FLOWER (UNII: O2T154T6OG) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) GREEN TEA LEAF (UNII: W2ZU1RY8B0) COMFREY ROOT (UNII: M9VVZ08EKQ) CARBOMER INTERPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 132584PQMO) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CAPRYLYL GLYCOL (UNII: 00YIU5438U) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49947-002-04 113.4 g in 1 BOTTLE; Type 0: Not a Combination Product 05/11/2023 2 NDC:49947-002-02 56.7 g in 1 BOTTLE; Type 0: Not a Combination Product 05/11/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/01/2012 Labeler - HUSH Anesthetic (012011309)