MULTI SYMPTOM COLD- acetaminophen, dextromethorphan, phenylephrine, guaifenesin tablet
Honeywell Safety Products USA, Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
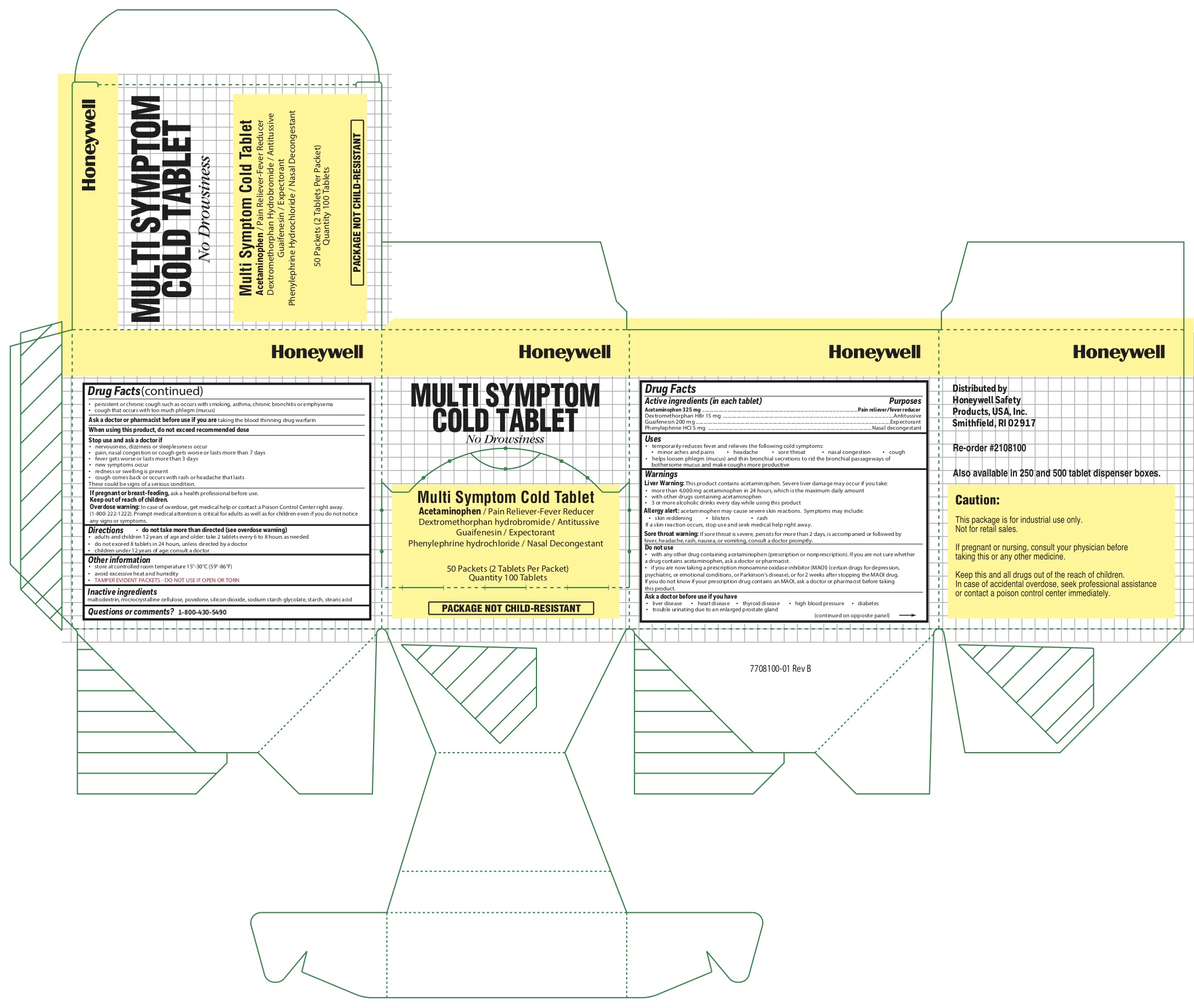
0498-1170 & 0498-1070: Multi Symptom Cold
Active Ingredients (in each tablet)
Acetaminophen 325 mg
Dextromethorphan HBr 15 mg
Guaifenesin 200 mg
Phenylephrine HCl 5 mg
Uses
temporarily reduces fever and relieves the following cold symptoms:
- minor aches and pains
- headache
- sore throat
- nasal congestion
- cough
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
Warnings
Liver Warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg acetaminophen in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen (prescription or nonprescription).
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If skin reaction occurs, stop use and seek medical help.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
• with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
• if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease
- heart disease
- thyroid disease
- high blood pressure
- diabetes
- trouble urinating due to an enlarged prostate
- persistant or chronic cough such as occurs with smoking, asthma, chronic bronchitis or emphysema
- cough that occurs with too much phlegm (mucus)
Stop use and ask a doctor if
- nervousness, dizziness or sleeplessness occur
- pain, nasal congestion or cough gets worse or lasts more than 7 days
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling i present
- cough comes back or occurs with rash or headache that lasts
These could be signs of a serious condition.
Directions
- do not take more than directed (see overdose warning)
- adults and children 12 years of age and older: take 2 tablets every 6 to 8 hours as needed
- do not exceed 8 tablets in 24 hours, unless directed by a doctor.
- children under 12 years of age: consult a doctor
Other Information
- store at controlled room temperature 15 o-30 oC (59 o-86 oF)
- avoid excessive heat and humidity
- TAMPER EVIDENT PACKETS- DO NOT USE IF OPEN OR TORN
| MULTI SYMPTOM COLD
acetaminophen, dextromethorphan, phenylephrine, guaifenesin tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| MULTI SYMPTOM COLD
acetaminophen, dextromethorphan, phenylephrine, guaifenesin tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Honeywell Safety Products USA, Inc (118768815) |
| Registrant - Honeywell Safety Products USA, Inc (118768815) |