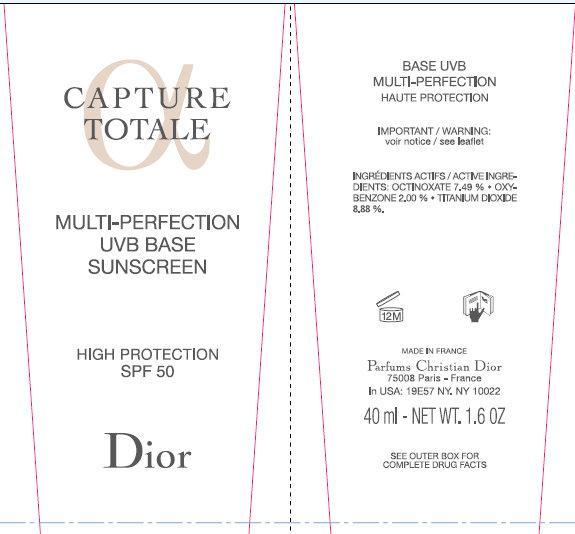
CD CAPTURE TOTALE MULTI-PERFECTION UVB BASE SUNSCREEN SPF 50- octinoxate, oxybenzone, titanium dioxide cream
Parfums Christian Dior
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CD CAPTURE TOTALE Multi-Perfection UVB Base Sunscreen SPF 50
Warnings
Skin Cancer/Skin Aging Alert: spending time in the sun increase your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn.
not skin cancer or early skin aging.
For external use only.
Directions
For sunscreen use
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
- Children under 6 months of age: ask a doctor.
Inactive ingredients
AQUA (WATER), C12-15 ALKYL BENZOATE, BUTYLENE GLYCOL, GLYCERIN, ISODODECANE, CI 77891 (TITANIUM DIOXIDE), POLYMETHYLSILSESQUIOXANE, SILICA, CETYL PEG/PPG-10/1 DIMETHICONE, PHENYL TRIMETHICONE, ISODODECANE, ALUMINUM STEARATE, POLYHYDROXYSTEARIC ACID, MAGNESIUM SULFATE, PHENOXYETHANOL, MICA, ALUMINA, SORBITAN SESQUIOLEATE, PARFUM (FRAGRANCE), ALCOHOL, CAPRYLYL GLYCOL, DISTEARDIMONIUM HECTORITE, TETRASODIUM EDTA, SODIUM MYRISTOYL GLUTAMATE, CI 77492 (IRON OXIDES), MALVA SYLVESTRIS (MALLOW) EXTRACT, CI 77491 (IRON OXIDES), PROPYLENE CARBONATE, DIMETHYLMETHOXY CHROMANOL, ADENOSINE, PLECTRANTHUS BARBATUS ROOT EXTRACT, ALUMINUM HYDROXIDE, BUTYLPHENYL METHYLPROPIONAL, FAEX (YEAST EXTRACT), BHT, LIMONENE, HYDROLYZED SOY PROTEIN, PENTYLENE GLYCOL, SODIUM METABISULFITE, ALPHAISOMETHYL IONONE, AFRAMOMUM ANGUSTIFOLIUM SEED EXTRACT, GERANIOL, KLUYVEROMYCES EXTRACT, OENOTHERA BIENNIS (EVENING PRIMROSE) ROOT EXTRACT, POLYGONUM BISTORTA ROOT EXTRACT, CITRONELLOL, CITRIC ACID, ETHYLHEXYLGLYCERIN, POTASSIUM SORBATE, SODIUM TOCOPHERYL PHOSPHATE.
Dior
For the first time, Dior combines the reinforced age-defying effectiveness of CAPTURE TOTALE with a protection against UVB damage and environmental aggressors in a new generation UVB Base.
New. The formula is based on an extraordinary combination of CAPTURE TOTALE age-defying ingredients and an ideal UVB protection for:
1. Intense correction of all visible signs of aging: wrinkles, loss of firmness and uneven skin tone.
2. Optimal protection of the mother cells* to prevent the appearance of age spots due to UVB rays.
With this maximum protection, skin becomes visibly smoother and more radiant. Day after day, the complexion becomes more even and radiants with new-found youth.
Daily protection UVB base SPF 50. Light, silky texture for ultimate comfort that absorbs instantly into the skin for maximum UVB protection and a flawless finish.
Apply in the morning to entire face after your regular CAPTURE TOTALE skincare ritual.
*
In vitro testing of ingredients: protection of cells from the basal layer of the epidermis that contains mother cells.
MADE IN FRANCE
Parfums Christian Dior
33, avenue Hoche - 75008 Paris - France
In USA: 19E57 NY. NY 10022
www.dior.com
| CD CAPTURE TOTALE MULTI-PERFECTION UVB BASE SUNSCREEN SPF 50
octinoxate, oxybenzone, titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Parfums Christian Dior (275252245) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Parfums Christian Dior | 396393746 | manufacture(61957-1461) | |