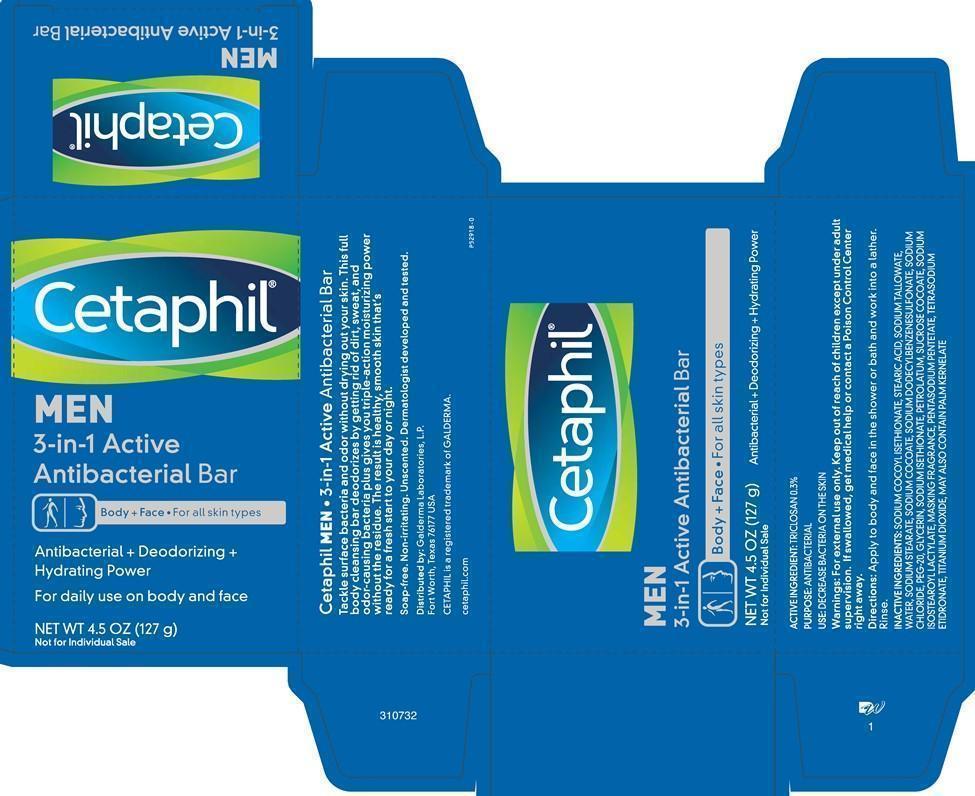
CETAPHIL MEN 3-IN-1 ACTIVE ANTIBACTERIAL BAR - triclosan soap
Galderma Laboratories, L.P.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Cetaphil MEN 3-in-1 Active Antibacterial Bar
INACTIVE INGREDIENTS:
SODIUM COCOYL ISETHIONATE, STEARIC ACID, SODIUM TALLOWATE, WATER, SODIUM STEARATE, SODIUM COCOATE, SODIUM DODECYLBENZENESULFONATE, SODIUM CHLORIDE, PEG-20, GLYCERIN, SODIUM ISETHIONATE, PETROLATUM, SUCROSE COCOATE, SODIUM ISOSTEAROYL LACTYLATE, MASKING FRAGRANCE, PENTASODIUM PENTETATE, TETRASODIUM ETIDRONATE, TITANIUM DIOXIDE, MAY ALSO CONTAIN PALM KERNELATE
Cetaphil®
MEN
3-in-1 Active Antibacterial Bar
Cetaphil®
MEN
3-in-1 Active Antibacterial Bar
Body + Face • For all skin types
Antibacterial + Deodorizing + Hydrating Power
For daily use on body and face
NET WT 4.5 OZ (127 g)
Not for Individual Sale
Cetaphil®
MEN • 3-in-1 Active Antibacterial Bar
Tackle surface bacteria and odor without drying out your skin. This full body cleansing bar deodorizes by getting rid of dirt, sweat, and odor-causing bacteria plus gives you triple-action moisturizing power without a residue. The result is healthy, smooth skin that's ready for a fresh start to your day or night.
Soap-free. Non-irritating. Unscented. Dermatologist developed and tested.
Distributed by: Galderma Laboratories, L.P.
Fort Worth, Texas 76177 USA
CETAPHIL is a registered trademark of GALDERMA.
cetaphil.com
P52918-0
ACTIVE INGREDIENT: TRICLOSAN 0.3%
PURPOSE: ANTIBACTERIAL
USE: DECREASE BACTERIA ON THE SKIN
Warnings: For external use only. Keep out of reach of children except under adult supervision. If swallowed, get medical help or contact a Poison Control Center right away.
Directions: Apply to body and face in the shower or bath and work into a lather. Rinse.
INACTIVE INGREDIENT: SODIUM COCOYL ISETHIONATE, STEARIC ACID, SODIUM TALLOWATE, WATER, SODIUM STEARATE, SODIUM COCOATE, SODIUM DODECYLBENZENESULFONATE, SODIUM CHLORIDE, PEG-20, GLYCERIN, SODIUM ISETHIONATE, PETROLATUM, SUCROSE COCOATE, SODIUM ISOSTEAROYL LACTYLATE, MASKING FRAGRANCE, PENTASODIUM PENTETATE, TETRASODIUM ETIDRONATE, TITANIUM DIOXIDE, MAY ALSO CONTAIN PALM KERNELATE
| CETAPHIL MEN 3-IN-1 ACTIVE ANTIBACTERIAL BAR
triclosan soap |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Galderma Laboratories, L.P. (047350186) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bradford Soap Works, Inc. | 001201045 | manufacture(0299-4100) | |