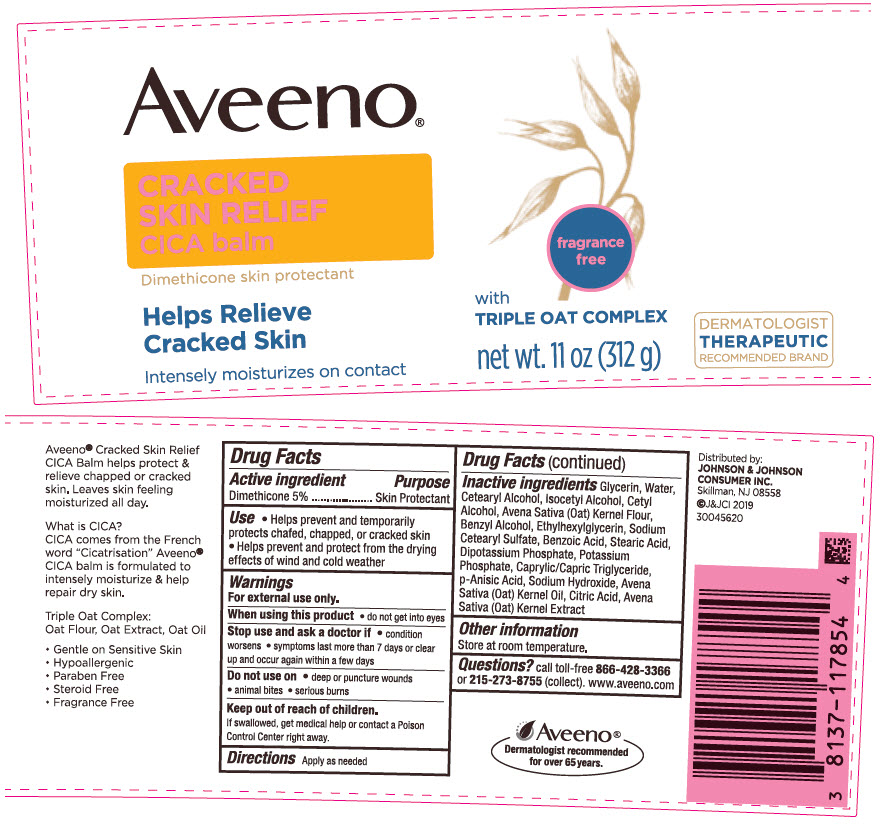
AVEENO CRACKED SKIN RELIEF CICA BALM- dimethicone emulsion
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Aveeno ® CRACKED SKIN RELIEF CICA balm
Use
- Helps prevent and temporarily protects chafed, chapped, or cracked skin
- Helps prevent and protect from the drying effects of wind and cold weather
Warnings
For external use only.
Inactive Ingredients
Glycerin, Water, Cetearyl Alcohol, Isocetyl Alcohol, Cetyl Alcohol, Avena Sativa (Oat) Kernel Flour, Benzyl Alcohol, Ethylhexylglycerin, Sodium Cetearyl Sulfate, Benzoic Acid, Stearic Acid, Dipotassium Phosphate, Potassium Phosphate, Caprylic/Capric Triglyceride, p-Anisic Acid, Sodium Hydroxide, Avena Sativa (Oat) Kernel Oil, Citric Acid, Avena Sativa (Oat) Kernel Extract
| AVEENO CRACKED SKIN RELIEF CICA BALM
dimethicone emulsion |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |
Revised: 10/2022
Document Id: eb8255a1-0c41-37d7-e053-2a95a90a70b1
Set id: 94e0e460-1f88-4b9e-9de3-8ab120e180e1
Version: 5
Effective Time: 20221020
Johnson & Johnson Consumer Inc.