Label: ENROSITE- enrofloxacin injection, solution
- NDC Code(s): 13985-709-20, 13985-709-50
- Packager: MWI/VetOne
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated September 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- CAUTION:
-
DESCRIPTION:
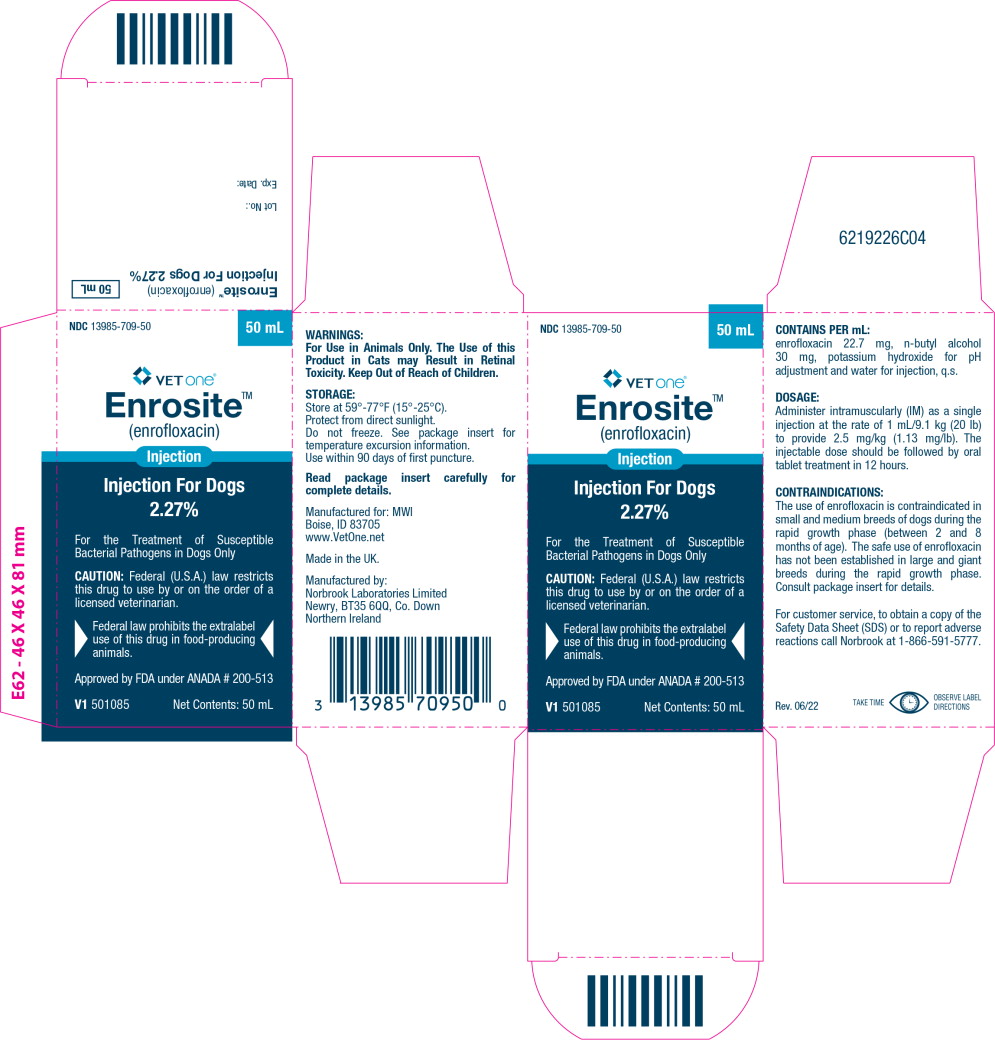
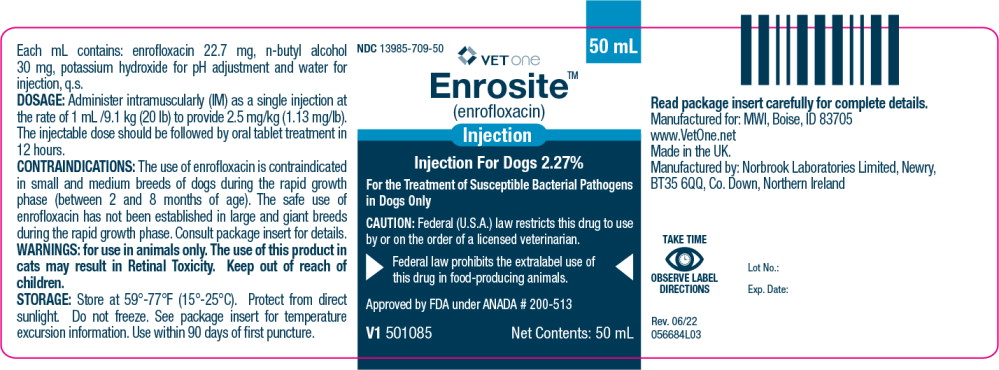
Enrofloxacin is a synthetic chemotherapeutic agent from the class of the quinolone carboxylic acid derivatives. It has antibacterial activity against a broad spectrum of Gram negative and Gram positive bacteria (See Tables I and II). Each mL of injectable solution contains: enrofloxacin 22.7 mg, n-butyl alcohol 30 mg, potassium hydroxide for pH adjustment and water for injection, q.s.
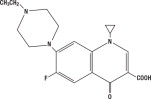
CHEMICAL NOMENCLATURE AND STRUCTURAL FORMULA:
1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1, 4-dihydro-4-oxo-3-quinolinecarboxylic acid.
-
ACTIONS:
Microbiology: Quinolone carboxylic acid derivatives are classified as DNA gyrase inhibitors. The mechanism of action of these compounds is very complex and not yet fully understood. The site of action is bacterial gyrase, a synthesis promoting enzyme. The effect of Escherichia coli is the inhibition of DNA synthesis through prevention of DNA supercoiling. Among other things, such compounds lead to the cessation of cell respiration and division. They may also interrupt bacterial membrane integrity.1
Enrofloxacin is bactericidal, with activity against both Gram negative and Gram positive bacteria. The minimum inhibitory concentrations (MICs) were determined for a series of 37 isolates representing 9 genera of bacteria from natural infections in dogs, selected principally because of resistance to one or more of the following antibiotics: ampicillin, cephalothin, colistin, chloramphenicol, erythromycin, gentamicin, kanamycin, penicillin, streptomycin, tetracycline, triple sulfa and sulfa/trimethoprim. The MIC values for enrofloxacin against these isolates are presented in Table I. Most strains of these organisms were found to be susceptible to enrofloxacin in vitro but the clinical significance has not been determined for some of the isolates.
The susceptibility of organisms to enrofloxacin should be determined using enrofloxacin 5 mcg disks. Specimens for susceptibility testing should be collected prior to the initiation of enrofloxacin therapy.
TABLE I – MIC Values for Enrofloxacin Against Canine Pathogens (Diagnostic laboratory isolates, 1984) Organisms Isolates MIC Range (mcg/mL) Bacteroides spp. 2 2 Bordatella bronchiseptica 3 0.125-0.5 Brucella canis 2 0.125-0.25 Clostridium perfringens 1 0.5 Escherichia coli 4 ≤0.016-0.031 Klebsiella spp. 10 0.031-0.5 Proteus mirabilis 6 0.062-0.125 Pseudomonas aeruginosa 4 0.5-8 Staphylococcus spp. 5 0.125 The inhibitory activity on 120 isolates of seven canine urinary pathogens was also investigated and is listed in Table II.
TABLE II – MIC Values for Enrofloxacin Against Canine Urinary Pathogens (Diagnostic laboratory isolates, 1985) Organisms Isolates MIC Range (mcg/mL) E. coli 30 0.06-2.0 P. mirabilis 20 0.125-2.0 K. pneumoniae 20 0.06-0.5 P. aeruginosa 10 1.0-8.0 Enterobacter spp. 10 0.06-1.0 Staph. (coag. +) 20 0.125-0.5 Strep. (alpa hemol.) 10 0.5-8.0 Distribution in the Body: Enrofloxacin penetrates into all canine tissues and body fluids. Concentrations of drug equal to or greater than the MIC for many pathogens (See Tables I, II and III) are reached in most tissues by two hours after dosing at 2.5 mg/kg and are maintained for 8-12 hours after dosing. Particularly high levels of enrofloxacin are found in urine.
A summary of the body fluid/tissue drug levels at 2 to 12 hours after dosing at 2.5 mg/kg is given in Table III.
TABLE III – Body Fluid/Tissue distribution of Enrofloxacin in Dogs Single Oral Dose = 2.5 mg/kg (1.13 mg/lb) Post-treatment Enrofloxacin Levels Canine (n=2) Body Fluids (mcg/mL) 2 Hr. 8 Hr. Urine 43.05 55.35 Eye Fluids 0.53 0.66 Whole Blood 1.01 0.36 Plasma 0.67 0.33 Tissues (mcg/g) Hematopoietic System Liver 3.02 1.36 Spleen 1.45 0.85 Bone Marrow 2.10 1.22 Lymph Node 1.32 0.91 Urogenital System Kidney 1.87 0.99 Bladder Wall 1.36 0.98 Testes 1.36 1.10 Prostate 1.36 2.20 Uterine Wall 1.59 0.29 Gastrointestinal and Cardiopulmonary Systems Lung 1.34 0.82 Heart 1.88 0.78 Stomach 3.24 2.16 Small Intestine 2.10 1.11 Other Fat 0.52 0.40 Skin 0.66 0.48 Muscle 1.62 0.77 Brain 0.25 0.24 Mammary Gland 0.45 0.21 Feces 1.65 9.97 Pharmacokinetics: In dogs, the absorption and elimination characteristics of the oral formulation are linear (plasma concentrations increase proportionally with dose) when enrofloxacin is administered at up to 11.5 mg/kg, twice daily.2 Approximately 80% of the orally administered dose enters the systemic circulation unchanged. The eliminating organs, based on the drug's body clearance time, can readily remove the drug with no indication that the eliminating mechanisms are saturated. The primary route of excretion is via the urine. The absorption and elimination characteristics beyond this point are unknown. Saturable absorption and/or elimination processes may occur at greater doses. When saturation of the absorption process occurs, the plasma concentration of the active moiety will be less than predicted, based on the concept of dose proportionality.
Following an oral dose in dogs of 2.5 mg/kg (1.13 mg/lb), enrofloxacin reached 50% of its maximum serum concentration in 15 minutes and peak serum level was reached in one hour. The elimination half-life in dogs is approximately 2 ½ -3 hours at that dose.
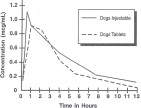
A graph indicating the mean serum levels following a dose of 2.5 mg/kg (1.13 mg/lb) in dogs (oral and intramuscular) is shown in Figure 1.
Figure 1 – Serum Concentrations of Enrofloxacin
Following a Single Oral or Intramuscular Dose at 2.5 mg/kg in Dogs.
Breakpoint: Based on pharmacokinetic studies of enrofloxacin in dogs after a single oral administration of 2.5 mg enrofloxacin/kg BW (i.e. half of the lowest-end single daily dose range) and the data listed in Tables I and II, the following breakpoints are recommended for canine isolates.
Zone Diameter (mm) MIC (μg/mL) Interpretation ≥ 21 ≤ 0.5 Susceptible (S) 18-20 1 Intermediate (I) ≤ 17 ≥ 2 Resistant (R) A report of “Susceptible” indicates that the pathogen is likely to be inhibited by generally achievable plasma levels. A report of “Intermediate” is a technical buffer and isolates falling into this category should be retested. Alternatively the organism may be successfully treated if the infection is in a body site where drug is physiologically concentrated. A report of “Resistant” indicates that the achievable drug concentrations are unlikely to be inhibitory and other therapy should be selected.
Standardized procedures require the use of laboratory control organisms for both standardized disk diffusion assays and standardized dilution assays.
The 5 μg enrofloxacin disk should give the following zone diameters and enrofloxacin powder should provide the following MIC values for reference strains.
QC Strain MIC (μg/mL) Zone Diameter (mm) E. coli ATCC 25922 0.008 - 0.03 32 - 40 P. aeruginosa ATCC 27853 1 - 4 15 - 19 S. aureus ATCC 25923 27 - 31 S. aureus ATCC 29213 0.03 - 0.12 - INDICATIONS:
-
EFFICACY CONFIRMATION:
Clinical efficacy was established in dermal infections (wounds and abscesses) associated with susceptible strains of Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Staphylococcus intermedius; respiratory infections (pneumonia, tonsillitis, rhinitis) associated with susceptible strains of Escherichia coli and Staphylococcus aureus and urinary cystitis associated with susceptible strains of Escherichia coli, Proteus mirabilis, and Staphylococcus aureus.
-
CONTRAINDICATIONS:
Enrofloxacin is contraindicated in dogs known to be hypersensitive to quinolones.
Based on the studies discussed under the section on Animal Safety Summary, the use of enrofloxacin is contraindicated in small and medium breeds of dogs during the rapid growth phase (between 2 and 8 months of age). The safe use of enrofloxacin has not been established in large and giant breeds during the rapid growth phase. Large breeds may be in this phase for up to one year of age and the giant breeds for up to 18 months. In clinical field trials utilizing a daily oral dose of 5.0 mg/kg, there were no reports of lameness or joint problems in any breed. However, controlled studies with histological examination of the articular cartilage have not been conducted in the large or giant breeds.
-
ADVERSE REACTIONS:
No drug-related side effects were reported in 122 clinical cases treated with an enrofloxacin injectable solution followed by enrofloxacin tablets at 5.0 mg/kg per day. To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Norbrook at 1-866-591-5777. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
-
ANIMAL SAFETY SUMMARY:
Adult dogs receiving enrofloxacin orally at a daily dosage rate of 52 mg/kg for 13 weeks had only isolated incidences of vomition and inappetence. Adult dogs receiving the tablet formulation for 30 consecutive days at a daily treatment of 25 mg/kg did not exhibit significant clinical signs nor were there effects upon the clinical chemistry, hematological or histological parameters. Daily doses of 125 mg/kg for up to 11 days induced vomition, inappetence, depression, difficult locomotion and death while adult dogs receiving 50 mg/kg/day for 14 days had clinical signs of vomition and inappetence.
Adult dogs dosed intramuscularly for three treatments at 12.5 mg/kg followed by 57 oral treatments at 12.5 mg/kg, all at 12 hour intervals, did not exhibit either significant clinical signs or effects upon the clinical chemistry, hematological or histological parameters.
Oral treatment of 15 to 28 week old growing puppies with daily dosage rates of 25 mg/kg has induced abnormal carriage of the carpal joint and weakness in the hindquarters. Significant improvement of clinical signs is observed following drug withdrawal. Microscopic studies have identified lesions of the articular cartilage following 30 day treatments at either 5, 15 or 25 mg/kg in this age group. Clinical signs of difficult ambulation or associated cartilage lesions have not been observed in 29 to 34 week old puppies following daily treatments of 25 mg/kg for 30 consecutive days nor in 2 week old puppies with the same treatment schedule.
Tests indicated no effect on circulating microfilariae or adult heartworms (Dirofilaria immitis) when dogs were treated at a daily dosage rate of 15 mg/kg for 30 days.
No effect on cholinesterase values was observed.
No adverse effects were observed on reproductive parameters when male dogs received 10 consecutive daily treatments of 15 mg/kg/day at 3 intervals (90, 45 and 14 days) prior to breeding or when female dogs received 10 consecutive daily treatments of 15 mg/kg/day at 4 intervals; between 30 and 0 days prior to breeding, early pregnancy (between 10th and 30th days), late pregnancy (between 40th and 60th days), and during lactation (the first 28 days).
-
DRUG INTERACTIONS:
Concomitant therapy with other drugs that are metabolized in the liver may reduce the clearance rates of the quinolone and the other drug.
Enrofloxacin has been administered to dogs at a daily dosage rate of 10 mg/kg concurrently with a wide variety of other health products including anthelmintics (praziquantel, febantel), insecticides (pyrethrins), heartworm preventatives (diethylcarbamazine) and other antibiotics (ampicillin, gentamicin sulfate, penicillin). No incompatibilities are known with other drugs at this time.
-
WARNINGS:
For use in animals only. The use of this product in cats may result in Retinal Toxicity. Keep out of reach of children.
Avoid contact with eyes. In case of contact, immediately flush eyes with copious amounts of water for 15 minutes. In case of dermal contact, wash skin with soap and water. Consult a physician if irritation persists following ocular or dermal exposure. Individuals with a history of hypersensitivity to quinolones should avoid this product. In humans, there is a risk of user photosensitization within a few hours after excessive exposure to quinolones. If excessive accidental exposure occurs, avoid direct sunlight.
For customer service, to obtain a copy of the Safety Data Sheet (SDS) or to report adverse reactions call Norbrook at 1-866-591-5777.
-
PRECAUTION:
Quinolone-class drugs should be used with caution in animals with known or suspected Central Nervous System (CNS) disorders. In such animals, quinolones have, in rare instances, been associated with CNS stimulation which may lead to convulsive seizures.
Quinolone-class drugs have been associated with cartilage erosions in weight-bearing joints and other forms of arthropathy in immature animals of various species.
The use of fluoroquinolones in cats has been reported to adversely affect the retina. Such products should be used with caution in cats.
-
DOSAGE AND ADMINISTRATION:
Enrosite Injection for Dogs may be used as the initial dose at 2.5 mg/kg.
It should be administered intramuscularly (IM) as a single dose, followed by initiation of enrofloxacin tablet therapy.
Enrosite Injection for Dogs may be administered as follows:
*The initial Enrosite Injection for Dogs administration should be followed 12 hours later by initiation of enrofloxacin tablet therapy.
Weight Of Animal Enrosite™ Injection for Dogs*
2.5 mg/kg9.1 kg (20 lb) 1.00 mL 27.2 kg (60 lb) 3.00 mL The lower limit of the dose range was based on efficacy studies in dogs where enrofloxacin was administered at 2.5 mg/kg twice daily. Target animal safety and toxicology studies were used to establish the upper limit of the dose range and treatment duration.
-
STORAGE:
Store at 59°-77°F (15°-25°C). Excursions permitted up to 86°F (30°C). Brief exposure to temperature up to 104°F (40°C) may be tolerated provided the mean kinetic temperature does not exceed 77°F (25°C); however, such exposure should be minimized. Protect from direct sunlight. Do not freeze.
Use within 90 days of first puncture.
- HOW SUPPLIED:
-
REFERENCES:
- Dougherty, T.J. and Saukkonen, J.J. Membrane Permeability Changes Associated with DNA Gyrase Inhibitors in Escherichia coli. Antimicrob. Agents and Chemoth., V. 28, Aug. 1985: 200-206.
- Walker, R.D., et al. Pharmacokinetic Evaluation of Enrofloxacin Administered Orally to Healthy Dogs. Am.J.Res., V. 53, No. 12, Dec. 1992: 2315-2319.
VETone®
Manufactured for: MWI
Boise, ID 83705www.VetOne.net
Made in the UK.
Manufactured by:
Norbrook Laboratories Limited
Newry, BT35 6QQ, Co. Down, Northern IrelandTAKE TIME
 OBSERVE LABEL
OBSERVE LABEL
DIRECTIONS058684I04
Label Rev. 06/22
-
Principal Display Panel – 50 mL Carton Label
NDC 13985-709-50
50 mL
VETone®
Enrosite™
(enrofloxacin)
InjectionInjection For Dogs
2.27%
For the Treatment of Susceptible
Bacterial Pathogens in Dogs OnlyCAUTION: Federal (U.S.A.) law restricts
this drug to use by or on the order of a
licensed veterinarian.Federal law prohibits the extralabel
use of this drug in food-producing
animals.Approved by FDA under ANADA # 200-513
V1 501085
Net Contents: 50 mL
-
Principal Display Panel – 50 mL Vial Label
NDC 13985-709-50
50 mL
VETone®
Enrosite™
(enrofloxacin)
InjectionInjection For Dogs 2.27%
For the Treatment of Susceptible Bacterial Pathogens
in Dogs OnlyCAUTION: Federal (U.S.A.) law restricts this drug to use
by or on the order of a licensed veterinarian.Federal law prohibits the extralabel use
of this drug in food-producing animals.Approved by FDA under ANADA # 200-513
V1 501085
Net Contents: 50 mL
-
INGREDIENTS AND APPEARANCE
ENROSITE
enrofloxacin injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:13985-709 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength enrofloxacin (UNII: 3DX3XEK1BN) (enrofloxacin - UNII:3DX3XEK1BN) enrofloxacin 22.7 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13985-709-20 1 in 1 CARTON 1 20 mL in 1 VIAL, GLASS 2 NDC:13985-709-50 1 in 1 CARTON 2 50 mL in 1 VIAL, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200513 05/06/2015 Labeler - MWI/VetOne (019926120) Registrant - Norbrook Laboratories Limited (211218325) Establishment Name Address ID/FEI Business Operations Norbrook Laboratories Limited 211218325 ANALYSIS, LABEL, MANUFACTURE, PACK