Label: RENAGEL tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 68151-4295-1 - Packager: Carilion Materials Management
- This is a repackaged label.
- Source NDC Code(s): 58468-0020
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 14, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Renagel safely and effectively. See full prescribing information for Renagel.
Renagel (sevelamer hydrochloride) Tablet for Oral use
Initial U.S. Approval: 2000RECENT MAJOR CHANGES
Contraindications (4) 03/2016 INDICATIONS AND USAGE
- Renagel® is a phosphate binder indicated for the control of serum phosphorus in patients with chronic kidney disease on dialysis. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- Tablets: 800 mg and 400 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Serious cases of dysphagia, bowel obstruction, and perforation have been associated with sevelamer use, some requiring hospitalization and surgery. (5.1)
ADVERSE REACTIONS
- The most common reasons for discontinuing treatment were gastrointestinal adverse reactions. (6.1)
- In a parallel design study of 12 weeks duration, treatment-emergent adverse reactions to Renagel Tablets in peritoneal dialysis patients included dyspepsia (12%), peritonitis (8%), diarrhea (5%), nausea (5%), constipation (4%), pruritus (4%), abdominal distension (3%), vomiting (3%), fatigue (3%), anorexia (3%), and arthralgia (3%). (6.1)
- Similar reactions at similar rates occurred in hemodialysis and peritoneal dialysis patients. (6.1)
- Cases of fecal impaction and, less commonly, ileus, bowel obstruction, and bowel perforation have been reported. (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Genzyme Corporation at 1-800-847-0069 and or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- When clinically significant drug interactions are expected, consider separation of the timing of administration and/or monitor clinical responses or blood levels of the concomitant medication. (7)
- Sevelamer did not alter the pharmacokinetics of digoxin, enalapril, iron, metoprolol, and warfarin. (7)
- Sevelamer has demonstrated interaction with ciprofloxacin, and mycophenolate mofetil, and therefore these drugs should be dosed separately from Renagel. (7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Gastrointestinal Adverse Events
5.2 Monitor Serum Chemistries
5.3 Monitor for Reduced Vitamins D, E, K (clotting factors) and Folic Acid Levels
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Active-Control, Cross-Over Study in Hemodialysis Patients
14.2 Active-Control, Parallel Study in Hemodialysis Patients
14.3 Active-Control, Parallel Study in Peritoneal Dialysis Patients
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Patients Not Taking a Phosphate Binder. The recommended starting dose of Renagel is 800 to 1600 mg, which can be administered as one or two 800 mg Renagel® Tablets, or two to four 400 mg Renagel® Tablets, with meals based on serum phosphorus level. Table 1 provides recommended starting doses of Renagel for patients not taking a phosphate binder.
Table 1: Starting Dose for Dialysis Patients Not Taking a Phosphate Binder Serum Phosphorus Renagel® 800 mg Renagel® 400 mg >5.5 and <7.5 mg/dL 1 tablet three times daily with meals 2 tablets three times daily with meals ≥7.5 and <9.0 mg/dL 2 tablets three times daily with meals 3 tablets three times daily with meals ≥9.0 mg/dL 2 tablets three times daily with meals 4 tablets three times daily with meals Patients Switching from Calcium Acetate. In a study in 84 CKD patients on hemodialysis, a similar reduction in serum phosphorus was seen with equivalent doses (approximately mg for mg) of Renagel and calcium acetate. Table 2 gives recommended starting doses of Renagel based on a patient's current calcium acetate dose.
Table 2: Starting Dose for Dialysis Patients Switching From Calcium Acetate to Renagel Calcium Acetate 667 mg
(Tablets per meal)Renagel® 800 mg
(Tablets per meal)Renagel® 400 mg
(Tablets per meal)1 tablet 1 tablet 2 tablets 2 tablets 2 tablets 3 tablets 3 tablets 3 tablets 5 tablets Dose Titration for All Patients Taking Renagel. Dosage should be adjusted based on the serum phosphorus concentration with a goal of lowering serum phosphorus to 5.5 mg/dL or less. The dose may be increased or decreased by one tablet per meal at two-week intervals as necessary. Table 3 gives a dose titration guideline. The average dose in a Phase 3 trial designed to lower serum phosphorus to 5.0 mg/dL or less was approximately three Renagel 800 mg tablets per meal. The maximum average daily Renagel dose studied was 13 grams.
Table 3: Dose Titration Guideline Serum Phosphorus Renagel® Dose >5.5 mg/dL Increase 1 tablet per meal at 2 week intervals 3.5–5.5 mg/dL Maintain current dose <3.5 mg/dL Decrease 1 tablet per meal - 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Gastrointestinal Adverse Events
Cases of dysphagia and esophageal tablet retention have been reported in association with use of the tablet formulation of sevelamer, some requiring hospitalization and intervention. Consider using sevelamer suspension in patients with a history of swallowing disorders.
Cases of bowel obstruction and perforation have also been reported with sevelamer use.
Patients with dysphagia, swallowing disorders, severe gastrointestinal (GI) motility disorders including severe constipation, or major GI tract surgery were not included in the Renagel clinical studies.
5.3 Monitor for Reduced Vitamins D, E, K (clotting factors) and Folic Acid Levels
In preclinical studies in rats and dogs, sevelamer hydrochloride reduced vitamins D, E, and K (coagulation parameters) and folic acid levels at doses of 6 to 10 times the recommended human dose. In short-term clinical trials, there was no evidence of reduction in serum levels of vitamins. However, in a one-year clinical trial, 25-hydroxyvitamin D (normal range 10 to 55 ng/mL) fell from 39 ± 22 ng/mL to 34 ± 22 ng/mL (p<0.01) with sevelamer hydrochloride treatment. Most (approximately 75%) patients in sevelamer hydrochloride clinical trials received vitamin supplements, which is typical of patients on dialysis.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug can not be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In a parallel design study of sevelamer hydrochloride with treatment duration of 52 weeks, adverse reactions reported for sevelamer hydrochloride (n=99) were similar to those reported for the active-control group (n=101). Overall adverse reactions among those treated with sevelamer hydrochloride occurring in >5% of patients included: vomiting (22%), nausea (20%), diarrhea (19%), dyspepsia (16%), abdominal pain (9%), flatulence (8%), and constipation (8%). A total of 27 patients treated with sevelamer and 10 patients treated with comparator withdrew from the study due to adverse reactions.
Based on studies of 8 to 52 weeks, the most common reason for withdrawal from Renagel was gastrointestinal adverse reactions (3%–16%).
In 143peritoneal dialysis patients studied for 12 weeks, most adverse reactions were similar to adverse reactions observed in hemodialysis patients. The most frequently occurring treatment-emergent serious adverse reaction was peritonitis (8 reactions in 8 patients [8%] in the sevelamer group and 2 reactions in 2 patients [4%] on active control). Thirteen patients (14%) in the sevelamer group and 9 patients (20%) in the active-control group discontinued, mostly for gastrointestinal adverse reactions. Patients on peritoneal dialysis should be closely monitored to ensure the reliable use of appropriate aseptic technique with the prompt recognition and management of any signs and symptoms associated with peritonitis.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of sevelamer hydrochloride (Renagel®): hypersensitivity, pruritus, rash, abdominal pain, fecal impaction and uncommon cases of ileus, intestinal obstruction, and intestinal perforation. Appropriate medical management should be given to patients who develop constipation or have worsening of existing constipation to avoid severe complications.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency or to establish a causal relationship to drug exposure.
-
7 DRUG INTERACTIONS
There are no empirical data on avoiding drug interactions between Renagel® and most concomitant oral drugs. For oral medication where a reduction in the bioavailability of that medication would have a clinically significant effect on its safety or efficacy (e.g., cyclosporine, tacrolimus, levothyroxine), consider separation of the timing of the administration of the two drugs [see Clinical Pharmacology (12.3)]. The duration of separation depends upon the absorption characteristics of the medication concomitantly administered, such as the time to reach peak systemic levels and whether the drug is an immediate-release or an extended-release product. Where possible consider monitoring clinical responses and/or blood levels of concomitant drugs that have a narrow therapeutic range.
Table 4: Sevelamer Drug Interactions Oral drugs for which sevelamer did not alter the pharmacokinetics when administered concomitantly Digoxin
Enalapril
Iron
Metoprolol
WarfarinOral drugs that have demonstrated interaction with sevelamer and are to be dosed separately from Renagel Dosing Recommendations Ciprofloxacin Take at least 2 hours before or 6 hours after sevelamer Mycophenolate mofetil Take at least 2 hours before sevelamer -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C: The effect of Renagel on the absorption of vitamins and other nutrients has not been studied in pregnant women. Requirements for vitamins and other nutrients are increased in pregnancy. In pregnant rats given doses of Renagel during organogenesis, reduced or irregular ossification of fetal bones, probably due to a reduced absorption of fat-soluble vitamin D, occurred. In pregnant rabbits given oral doses of Renagel by gavage during organogenesis, an increase of early resorptions occurred [see Nonclinical Toxicology (13.1)].
8.2 Labor and Delivery
No Renagel treatment-related effects on labor and delivery were seen in animal studies. The effects of Renagel on labor and delivery in humans are not known [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and efficacy of Renagel has not been established in pediatric patients.
8.5 Geriatric Use
Clinical studies of Renagel did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range.
-
10 OVERDOSAGE
Renagel has been given to normal healthy volunteers in doses of up to 14 grams per day for eight days with no adverse effects. Renagel has been given in average doses up to 13 grams per day to hemodialysis patients. There are no reports of overdosage with Renagel in patients. Since Renagel is not absorbed, the risk of systemic toxicity is low.
-
11 DESCRIPTION
The active ingredient in Renagel Tablets is sevelamer hydrochloride, a polymeric amine that binds phosphate and is meant for oral administration. Sevelamer hydrochloride is poly(allylamine hydrochloride) crosslinked with epichlorohydrin in which 40% of the amines are protonated. It is known chemically as poly(allylamine-co-N,N'-diallyl-1,3-diamino-2-hydroxypropane) hydrochloride. Sevelamer hydrochloride is hydrophilic, but insoluble in water. The structure is represented in Figure 1.
Figure 1: Chemical Structure of Sevelamer Hydrochloride
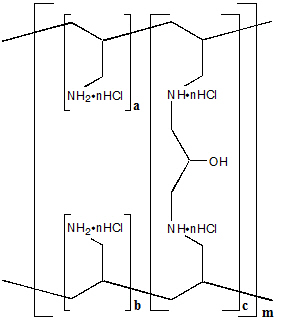
a, b = number of primary amine groups a + b = 9 c = number of crosslinking groups c = 1 n = fraction of protonated amines n = 0.4 m = large number to indicate extended polymer network The primary amine groups shown in the structure are derived directly from poly(allylamine hydrochloride). The crosslinking groups consist of two secondary amine groups derived from poly(allylamine hydrochloride) and one molecule of epichlorohydrin.
Renagel® Tablets: Each film-coated tablet of Renagel contains either 800 mg or 400 mg of sevelamer hydrochloride on an anhydrous basis. The inactive ingredients are hypromellose, diacetylated monoglyceride, colloidal silicon dioxide, and stearic acid. The tablet imprint contains iron oxide black ink.
-
12 CLINICAL PHARMACOLOGY
Patients with chronic kidney disease (CKD) on dialysis retain phosphorus and can develop hyperphosphatemia. High serum phosphorus can precipitate serum calcium resulting in ectopic calcification. When the product of serum calcium and phosphorus concentrations (Ca × P) exceeds 55 mg2/dL2, there is an increased risk that ectopic calcification will occur. Hyperphosphatemia plays a role in the development of secondary hyperparathyroidism in renal insufficiency.
Treatment of hyperphosphatemia includes reduction in dietary intake of phosphate, inhibition of intestinal phosphate absorption with phosphate binders, and removal of phosphate with dialysis. Renagel taken with meals has been shown to decrease serum phosphorus concentrations in patients with CKD who are on dialysis.
12.1 Mechanism of Action
Renagel contains sevelamer hydrochloride, a non-absorbed binding crosslinked polymer. It contains multiple amines separated by one carbon from the polymer backbone. These amines exist in a protonated form in the intestine and interact with phosphate molecules through ionic and hydrogen bonding. By binding phosphate in the dietary tract and decreasing absorption, sevelamer hydrochloride lowers the phosphate concentration in the serum.
12.2 Pharmacodynamics
In addition to effects on serum phosphate levels, sevelamer hydrochloride has been shown to bind bile acids in vitro and in vivo in experimental animal models. Bile acid binding by ion exchange resins is a well-established method of lowering blood cholesterol. Because sevelamer binds bile acids, it may interfere with normal fat absorption and thus may reduce absorption of fat-soluble vitamins such as A, D, and K. In clinical trials of sevelamer hydrochloride, both the mean total and LDL cholesterol declined by 15% to 31%. This effect is observed after 2 weeks. Triglycerides, HDL cholesterol, and albumin did not change.
12.3 Pharmacokinetics
A mass balance study using 14C-sevelamer hydrochloride in 16 healthy male and female volunteers showed that sevelamer hydrochloride is not systemically absorbed. No absorption studies have been performed in patients with renal disease.
Drug Interactions
In vivo
Sevelamer carbonate has been studied in human drug-drug interaction studies (9.6 grams once daily with a meal) with warfarin and digoxin. Sevelamer hydrochloride, which contains the same active moiety as sevelamer carbonate, has been studied in human drug-drug interaction studies (2.4–2.8 grams single dose or three times daily with meals or two times daily without meals) with ciprofloxacin, digoxin, enalapril, iron, metoprolol, mycophenolate mofetil and warfarin.
Coadministered single dose of 2.8 grams of sevelamer hydrochloride in fasted state decreased the bioavailability of ciprofloxacin by approximately 50% in healthy subjects.
Concomitant administration of sevelamer and mycophenolate mofetil in adult and pediatric patients decreased the mean MPA Cmax and AUC0–12h by 36% and 26%, respectively.
Sevelamer carbonate or sevelamer hydrochloride did not alter the pharmacokinetics of a single dose of enalapril, digoxin, iron, metoprolol and warfarin when coadministered.
During postmarketing experience, cases of increased thyroid stimulating hormone (TSH) levels have been reported in patients coadministered sevelamer hydrochloride and levothyroxine. Reduction in concentrations of cyclosporine and tacrolimus leading to dose increases has also been reported in transplant patients when coadministered with sevelamer hydrochloride without any clinical consequences (e.g., graft rejection). The possibility of an interaction cannot be excluded with these drugs.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Standard lifetime carcinogenicity bioassays were conducted in mice and rats. Rats were given sevelamer hydrochloride by diet at 0.3, 1, or 3 g/kg/day. There was an increased incidence of urinary bladder transitional cell papilloma in male rats of the high-dose group (human equivalent dose twice the maximum clinical trial dose of 13 g). Mice received dietary administration of sevelamer hydrochloride at doses of up to 9 g/kg/day (human equivalent dose 3 times the maximum clinical trial dose). There was no increased incidence of tumors observed in mice.
In an in vitro mammalian cytogenetic test with metabolic activation, sevelamer hydrochloride caused a statistically significant increase in the number of structural chromosome aberrations. Sevelamer hydrochloride was not mutagenic in the Ames bacterial mutation assay.
Sevelamer hydrochloride did not impair the fertility of male or female rats in a dietary administration study in which the females were treated from 14 days prior to mating through gestation and the males were treated for 28 days prior to mating. The highest dose in this study was 4.5 g/kg/day (human equivalent dose 3 times the maximum clinical trial dose of 13 g).
In pregnant rats given dietary doses of 0.5, 1.5 or 4.5 g/kg/day of sevelamer hydrochloride during organogenesis, reduced or irregular ossification of fetal bones, probably due to a reduced absorption of fat-soluble vitamin D, occurred in mid and high-dose groups (human equivalent doses less than the maximum clinical trial dose of 13 g). In pregnant rabbits given oral doses of 100, 500, or 1000 mg/kg/day of sevelamer hydrochloride by gavage during organogenesis, an increase of early resorptions occurred in the high-dose group (human equivalent dose twice the maximum clinical trial dose).
-
14 CLINICAL STUDIES
The ability of Renagel to lower serum phosphorus in CKD patients on dialysis was demonstrated in six clinical trials: one double-blind placebo-controlled 2-week study (Renagel N=24); two open-label uncontrolled 8-week studies (Renagel N=220) and three active-controlled open-label studies with treatment durations of 8 to 52 weeks (Renagel N=256). Three of the active-controlled studies are described here. One is a crossover study with two 8-week periods comparing Renagel to an active control. The second is a 52-week parallel study comparing Renagel with active control. The third is a 12-week parallel study comparing Renagel and active control in peritoneal dialysis patients.
14.1 Active-Control, Cross-Over Study in Hemodialysis Patients
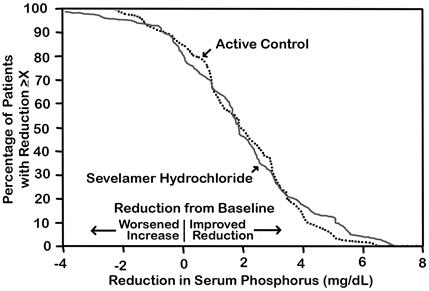
Eighty-four CKD patients on hemodialysis who were hyperphosphatemic (serum phosphorus >6.0 mg/dL) following a two-week phosphate-binder washout period received Renagel and active control for eight weeks each in random order. Treatment periods were separated by a two-week phosphate-binder washout period. Patients started on treatment three times per day with meals. Over each eight-week treatment period, at three separate time points the dose of Renagel could be titrated up 1 capsule or tablet per meal (3 per day) to control serum phosphorus, the dose of active control could also be altered to attain phosphate control. Both treatments significantly decreased mean serum phosphorus by about 2 mg/dL (Table 5).
Table 5: Mean Serum Phosphorus (mg/dL) at Baseline and Endpoint Renagel®
(N=81)Active-Control
(N=83)- *
- p<0.0001, within treatment group comparison
Baseline at End of Washout 8.4 8.0 Endpoint 6.4 5.9 Change from Baseline at Endpoint
(95% Confidence Interval)-2.0*
(-2.5, -1.5)-2.1*
(-2.6, -1.7)The distribution of responses is shown in Figure 2. The distributions are similar for sevelamer hydrochloride and active control. The median response is a reduction of about 2 mg/dL in both groups. About 50% of subjects have reductions between 1 and 3 mg/dL.
Figure 2: Percentage of Patients (Y-axis) Attaining a Phosphorus Reduction from Baseline (mg/dL) at Least as Great as the Value of the X-axis.
Average daily Renagel dose at the end of treatment was 4.9 g (range of 0.0 to 12.6 g).
14.2 Active-Control, Parallel Study in Hemodialysis Patients
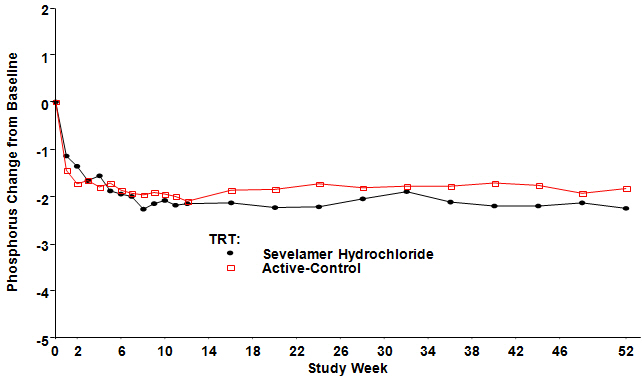
Two hundred CKD patients on hemodialysis who were hyperphosphatemic (serum phosphorus >5.5 mg/dL) following a two-week phosphate-binder washout period were randomized to receive Renagel 800 mg tablets (N=99) or an active control (N=101). The two treatments produced similar decreases in serum phosphorus. At week 52, using last observation carried forward, Renagel and active control both significantly decreased mean serum phosphorus (Table 6).
Table 6: Mean Serum Phosphorus (mg/dL) and Ion Product at Baseline and Change from Baseline to End of Treatment Renagel®
(N=94)Active-Control
(N=98)Phosphorus Baseline 7.5 7.3 Change from Baseline at Endpoint -2.1 -1.8 Ca × Phosphorus Ion Product Baseline 70.5 68.4 Change from Baseline at Endpoint -19.4 -14.2 Sixty-one percent of Renagel patients and 73% of the control patients completed the full 52 weeks of treatment.
Figure 3, a plot of the phosphorus change from baseline for the completers, illustrates the durability of response for patients who are able to remain on treatment.
Figure 3: Mean Phosphorus Change from Baseline for Patients who Completed 52 Weeks of Treatment
Average daily Renagel dose at the end of treatment was 6.5 g (range of 0.8 to 13 g).
14.3 Active-Control, Parallel Study in Peritoneal Dialysis Patients
One hundred and forty-three patients on peritoneal dialysis, who were hyperphosphatemic (serum phosphorus >5.5 mg/dL) following a two-week phosphate-binder washout period, were randomized to receive Renagel® (N=97) or active control (N=46) open label for 12 weeks. Average daily Renagel dose at the end of treatment was 5.9 g (range 0.8 to 14.3 g). There were statistically significant changes in serum phosphorus (p<0.001) for Renagel (-1.6 mg/dL from baseline of 7.5 mg/dL), similar to the active control.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- SPL UNCLASSIFIED SECTION
- RENAGEL TABLET
-
INGREDIENTS AND APPEARANCE
RENAGEL
renagel tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68151-4295(NDC:58468-0020) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SEVELAMER HYDROCHLORIDE (UNII: GLS2PGI8QG) (SEVELAMER - UNII:941N5DUU5C) SEVELAMER HYDROCHLORIDE 400 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) 1.6 mg STEARIC ACID (UNII: 4ELV7Z65AP) 1.6 mg WATER (UNII: 059QF0KO0R) 32.3 mg HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) 8.35 mg HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) 8.35 mg FERROSOFERRIC OXIDE (UNII: XM0M87F357) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ISOPROPYL ALCOHOL (UNII: ND2M416302) Product Characteristics Color WHITE (WHITE) Score no score Shape OVAL (OVAL) Size 15mm Flavor Imprint Code RENAGEL;400 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68151-4295-1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 12/28/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021179 06/06/2008 Labeler - Carilion Materials Management (079239644) Establishment Name Address ID/FEI Business Operations Carilion Materials Management 079239644 REPACK(68151-4295)


