TALACEN- pentazocine hydrochloride and acetaminophen tablet
sanofi-aventis U.S. LLC
----------
TALACEN®
Pentazocine hydrochloride, USP,
and acetaminophen, USP.
DESCRIPTION
TALACEN is a combination of pentazocine hydrochloride, USP, equivalent to 25 mg base and acetaminophen, USP, 650 mg.
Pentazocine is a member of the benzazocine series (also known as the benzomorphan series). Chemically, pentazocine is (2R*, 6R*, 11R*)1,2,3,4,5,6-hexahydro-6,11-dimethyl-3-(3-methyl-2-butenyl)-2,6-methano-3-benzazocin-8-ol, a white, crystalline substance soluble in acidic aqueous solutions, and has the following structural formula:
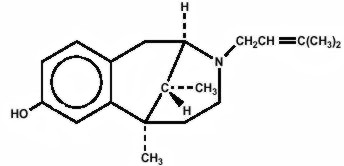
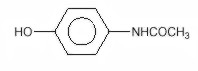
Chemically, acetaminophen is Acetamide, N-(4-hydroxyphenyl)-, and has the following structural formula:
Pentazocine is an analgesic and acetaminophen is an analgesic and antipyretic.
Inactive Ingredients: Colloidal Silicon Dioxide, FD&C Blue #1, Gelatin, Microcrystalline Cellulose, Potassium Sorbate, Pregelatinized Starch, Sodium Lauryl Sulfate, Sodium Metabisulfite, Sodium Starch Glycolate, Stearic Acid.
CLINICAL PHARMACOLOGY
TALACEN is an analgesic possessing antipyretic actions.
Pentazocine is an analgesic with agonist/antagonist action which when administered orally is approximately equivalent on a mg for mg basis in analgesic effect to codeine.
Acetaminophen is an analgesic and antipyretic.
Onset of significant analgesia with pentazocine usually occurs between 15 and 30 minutes after oral administration, and duration of action is usually three hours or longer. Onset and duration of action and the degree of pain relief are related both to dose and the severity of pretreatment pain. Pentazocine weakly antagonizes the analgesic effects of morphine, meperidine, and phenazocine; in addition, it produces incomplete reversal of cardiovascular, respiratory, and behavioral depression induced by morphine and meperidine. Pentazocine has about 1/50 the antagonistic activity of nalorphine. It also has sedative activity.
Pentazocine is well absorbed from the gastrointestinal tract. Plasma levels closely correspond to the onset, duration, and intensity of analgesia. The time to mean peak concentration in 24 normal volunteers was 1.7 hours (range 0.5 to 4 hours) after oral administration and the mean plasma elimination half-life was 3.6 hours (range 1.5 to 10 hours).
The action of pentazocine is terminated for the most part by biotransformation in the liver with some free pentazocine excreted in the urine. The products of the oxidation of the terminal methyl groups and glucuronide conjugates are excreted by the kidney. Elimination of approximately 60% of the total dose occurs within 24 hours. Pentazocine passes the placental barrier.
Onset of significant analgesic and antipyretic activity of acetaminophen when administered orally occurs within 30 minutes and is maximal at approximately 2 1/2 hours. The pharmacological mode of action of acetaminophen is unknown at this time.
Acetaminophen is rapidly and almost completely absorbed from the gastrointestinal tract. In 24 normal volunteers the time to mean peak plasma concentration was 1 hour (range 0.25 to 3 hours) after oral administration and the mean plasma elimination half-life was 2.8 hours (range 2 to 4 hours).
The effect of pentazocine on acetaminophen plasma protein binding or vice versa has not been established. For acetaminophen there is little or no plasma protein binding at normal therapeutic doses. When toxic doses of acetaminophen are ingested and drug plasma levels exceed 90 mcg/mL, plasma binding may vary from 8% to 43%.
Acetaminophen is conjugated in the liver with glucuronic acid and to a lesser extent with sulfuric acid. Approximately 80% of acetaminophen is excreted in the urine after conjugation and about 3% is excreted unchanged. The drug is also conjugated to a lesser extent with cysteine and additionally metabolized by hydroxylation.
If TALACEN is taken every 4 hours over an extended period of time, accumulation of pentazocine and to a lesser extent, acetaminophen, may occur.
CONTRAINDICATIONS
TALACEN should not be administered to patients who are hypersensitive to either pentazocine or acetaminophen.
WARNINGS
Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
Head Injury and Increased Intracranial Pressure
As in the case of other potent analgesics, the potential of pentazocine for elevating cerebrospinal fluid pressure may be attributed to CO2 retention due to the respiratory depressant effects of the drug. These effects may be markedly exaggerated in the presence of head injury, other intracranial lesions, or a preexisting increase in intracranial pressure. Furthermore, pentazocine can produce effects which may obscure the clinical course of patients with head injuries. In such patients, TALACEN must be used with extreme caution and only if its use is deemed essential.
Acute CNS Manifestations
Patients receiving therapeutic doses of pentazocine have experienced hallucinations (usually visual), disorientation, and confusion which have cleared spontaneously within a period of hours. The mechanism of this reaction is not known. Such patients should be closely observed and vital signs checked. If the drug is reinstituted, it should be done with caution since these acute CNS manifestations may recur.
There have been instances of psychological and physical dependence on parenteral pentazocine in patients with a history of drug abuse, and rarely, in patients without such a history. (See DRUG ABUSE AND DEPENDENCE.)
Due to the potential for increased CNS depressant effects, alcohol should be used with caution in patients who are currently receiving pentazocine.
Pentazocine may precipitate opioid abstinence symptoms in patients receiving courses of opiates for pain relief.
PRECAUTIONS
In prescribing TALACEN for chronic use, the physician should take precautions to avoid increases in dose by the patient.
Myocardial Infarction
As with all drugs, TALACEN should be used with caution in patients with myocardial infarction who have nausea or vomiting.
Certain Respiratory Conditions
Although respiratory depression has rarely been reported after oral administration of pentazocine, the drug should be administered with caution to patients with respiratory depression from any cause, severely limited respiratory reserve, severe bronchial asthma and other obstructive respiratory conditions, or cyanosis.
Impaired Renal or Hepatic Function
Decreased metabolism of the drug by the liver in extensive liver disease may predispose to accentuation of side effects. Although laboratory tests have not indicated that pentazocine causes or increases renal or hepatic impairment, the drug should be administered with caution to patients with such impairment.
Since acetaminophen is metabolized by the liver, the question of the safety of its use in the presence of liver disease should be considered.
Biliary Surgery
Narcotic drug products are generally considered to elevate biliary tract pressure for varying periods following their administration. Some evidence suggests that pentazocine may differ from other marketed narcotics in this respect (i.e., it causes little or no elevation in biliary tract pressures). The clinical significance of these findings, however, is not yet known.
CNS Effect
Caution should be used when TALACEN is administered to patients prone to seizures; seizures have occurred in a few such patients in association with the use of pentazocine although no cause and effect relationship has been established.
Information for Patients
Since sedation, dizziness, and occasional euphoria have been noted, ambulatory patients should be warned not to operate machinery, drive cars, or unnecessarily expose themselves to hazards. Pentazocine may cause physical and psychological dependence when taken alone and may have additive CNS depressant properties when taken in combination with alcohol or other CNS depressants.
Drug Interactions
Pentazocine is a mild narcotic antagonist. Some patients previously given narcotics, including methadone for the daily treatment of narcotic dependence, have experienced withdrawal symptoms after receiving pentazocine.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis, mutagenesis, and impairment of fertility studies have not been done with this combination product.
Pentazocine, when administered orally or parenterally, had no adverse effect on either the reproductive capabilities or the course of pregnancy in rabbits and rats. Embryotoxic effects on the fetuses were not shown.
The daily administration of 4 mg/kg to 20 mg/kg pentazocine subcutaneously to female rats during a 14 day pre-mating period and until the 13th day of pregnancy did not have any adverse effects on the fertility rate.
There is no evidence in long-term animal studies to demonstrate that pentazocine is carcinogenic.
Pregnancy Category C
Animal reproduction studies have not been conducted with TALACEN. It is also not known whether TALACEN can cause fetal harm when administered to pregnant women or can affect reproduction capacity. TALACEN should be given to pregnant women only if clearly needed. However, animal reproduction studies with pentazocine have not demonstrated teratogenic or embryotoxic effects.
Nonteratogenic Effects
There has been no experience in this regard with the combination pentazocine and acetaminophen. However, there have been rare reports of possible abstinence syndromes in newborns after prolonged use of pentazocine during pregnancy.
Labor and Delivery
Patients receiving pentazocine during labor have experienced no adverse effects other than those that occur with commonly used analgesics. TALACEN should be used with caution in women delivering premature infants. The effect of TALACEN on the mother and fetus, the duration of labor or delivery, the possibility that forceps delivery or other intervention or resuscitation of the newborn may be necessary, or the effect of TALACEN, on the later growth, development, and functional maturation of the child are unknown at the present time.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when TALACEN is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 12 have not been established.
Geriatric Use
Clinical studies of TALACEN did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
Clinical experience with TALACEN has been insufficient to define all possible adverse reactions with this combination. However, reactions reported after oral administration of pentazocine hydrochloride in 50 mg dosage include gastrointestinal: nausea, vomiting, infrequently constipation; and rarely abdominal distress, anorexia, diarrhea. CNS effects: dizziness, lightheadedness, hallucinations, sedation, euphoria, headache, confusion, disorientation; infrequently weakness, disturbed dreams, insomnia, syncope, visual blurring and focusing difficulty, depression; and rarely tremor, irritability, excitement, tinnitus. Autonomic: sweating; infrequently flushing; and rarely chills. Allergic: infrequently rash; and rarely urticaria, edema of the face. Cardiovascular: infrequently decrease in blood pressure, tachycardia. Hematologic: rarely depression of white blood cells (especially granulocytes), which is usually reversible, moderate transient eosinophilia. Other: rarely respiratory depression, urinary retention, paresthesia, serious skin reactions, including erythema multiforme, Stevens-Johnson Syndrome, toxic epidermal necrolysis, and in one instance, an apparent anaphylactic reaction has been reported.
Numerous clinical studies have shown that acetaminophen, when taken in recommended doses, is relatively free of adverse effects in most age groups, even in the presence of a variety of disease states.
A few cases of hypersensitivity to acetaminophen have been reported, as manifested by skin rashes, thrombocytopenic purpura, rarely hemolytic anemia and agranulocytosis.
Occasional individuals respond to ordinary doses with nausea and vomiting and diarrhea.
DRUG ABUSE AND DEPENDENCE
Abuse and Dependence
There have been some reports of dependence and of withdrawal symptoms with orally administered pentazocine. There have been recorded instances of psychological and physical dependence in patients using parenteral pentazocine. Abrupt discontinuance following the extended use of parenteral pentazocine has resulted in withdrawal symptoms. Patients with a history of drug dependence should be under close supervision while receiving TALACEN. There have been rare reports of possible abstinence syndromes in newborns after prolonged use of pentazocine during pregnancy.
Some tolerance to the analgesic and subjective effects of pentazocine develops with frequent and repeated use.
Drug addicts who are given closely spaced doses of pentazocine (e.g., 60 mg to 90 mg every 4 hours) develop physical dependence which is demonstrated by abrupt withdrawal or by administration of naloxone. The withdrawal symptoms exhibited after chronic doses of more than 500 mg of pentazocine per day have similar characteristics, but to a lesser degree, of opioid withdrawal and may be associated with drug seeking behavior.
OVERDOSAGE
Manifestations
Clinical experience with TALACEN has been insufficient to define the signs of overdosage with this product. It may be assumed that signs and symptoms of TALACEN overdose would be a combination of those observed with pentazocine overdose and acetaminophen overdose.
For pentazocine alone in single doses above 60 mg there have been reports of the occurrence of nalorphine-like psychotomimetic effects such as anxiety, nightmares, strange thoughts, and hallucinations. Marked respiratory depression associated with increased blood pressure and tachycardia have also resulted from excessive doses as have dizziness, nausea, vomiting, lethargy, and paresthesias. The respiratory depression is antagonized by naloxone (see Treatment).
In acute acetaminophen overdosage, dose-dependent, potentially fatal hepatic necrosis is the most serious adverse effect. Renal tubular necrosis, hypoglycemic coma, and thrombocytopenia may also occur.
In adults, a single dose of 10 g to 15 g (200 mg/kg to 250 mg/kg) of acetaminophen may cause hepatotoxicity. A dose of 25 g or more is potentially fatal. The potential seriousness of the intoxication may not be evident during the first two days of acute acetaminophen poisoning. During the first 24 hours, nausea, vomiting, anorexia, and abdominal pain occur. These may persist for a week or more. Liver injury may become evident the second day, initial signs being elevation of serum transaminase and lactic dehydrogenase activity, increased serum bilirubin concentration, and prolongation of prothrombin time. Serum albumin concentration and alkaline phosphatase activity may remain normal. The hepatotoxicity may lead to encephalopathy, coma, and death. Transient azotemia is evident in a majority of patients and acute renal failure occurs in some.
There have been reports of glycosuria and impaired glucose tolerance, but hypoglycemia may also occur. Metabolic acidosis and metabolic alkalosis have been reported. Cerebral edema and non-specific myocardial depression have also been noted. Biopsy reveals centrolobular necrosis with sparing of the periportal area. The hepatic lesions are reversible over a period of weeks or months in nonfatal cases.
The severity of the liver injury can be determined by measurement of the plasma halftime of acetaminophen during the first day of acute poisoning. If the halftime exceeds 4 hours, hepatic necrosis is likely and if the halftime is greater than 12 hours, hepatic coma will probably occur. Only minimal liver damage has developed when the serum concentration was below 120 mcg/mL at 12 hours after ingestion of the drug. If serum bilirubin concentration is greater than 4 mg/100 mL during the first 5 days, encephalopathy may occur.
The seven day oral LD50 value for TALACEN in mice is 3,570 mg/kg.
Treatment
Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated. Assisted or controlled ventilation should also be considered. For respiratory depression due to overdosage or unusual sensitivity to TALACEN, parenteral naloxone is a specific and effective antagonist.
The toxic effects of acetaminophen may be prevented or minimized by antidotal therapy with N acetylcysteine. In order to obtain the best possible results, N-acetylcysteine should be administered as soon as possible.
Vigorous supportive therapy is required in severe intoxication. Procedures to limit the continuing absorption of the drug must be readily performed since the hepatic injury is dose dependent and occurs early in the course of intoxication. Induction of vomiting or gastric lavage, followed by oral administration of activated charcoal should be done in all cases.
If hemodialysis can be initiated within the first 12 hours, it is advocated for patients with a plasma acetaminophen concentration exceeding 120 mcg/mL at 4 hours after ingestion of the drug.
DOSAGE AND ADMINISTRATION
Adult
The usual adult dose is 1 caplet every 4 hours as needed for pain relief, up to a maximum of 6 caplets per day.
The usual duration of therapy is dependent upon the condition being treated but in any case should be reviewed regularly by the physician. The effect of meals on the rate and extent of bioavailability of both pentazocine and acetaminophen has not been documented.
HOW SUPPLIED
Talacen is available for oral administration as a pale blue, scored caplet embossed with "Winthrop" on one side and "T37" on the other side.
Bottles of 100 (NDC 0024-1937-04).
| TALACEN
pentazocine hydrochloride and acetaminophen tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - sanofi-aventis U.S. LLC (824676584) |
