ANBESOL REGULAR STRENGTH- benzocaine gel
ANBESOL REGULAR STRENGTH- benzocaine solution
ANBESOL MAXIMUM STRENGTH- benzocaine gel
ANBESOL MAXIMUM STRENGTH- benzocaine solution
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
USES
- •
- temporarily relieves pain associated with the following mouth and gum irritations:
- •
- toothache
- •
- sore gums
- •
- canker sores
- •
- braces
- •
- minor dental procedures
- •
- dentures
WARNINGS
METHEMOGLOBINEMIA WARNING
Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried in the blood. This can occur even if you have used this product before. Stop use and seek immediate medical attention if you or a child in your care develops:
- •
- pale, gray, or blue colored skin (cyanosis)
- •
- headache
- •
- rapid heart rate
- •
- shortness of breath
- •
- dizziness or lightheadedness
- •
- fatigue or lack of energy
Allergy alert
Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other "caine" anesthetics
When using this product
- •
- avoid contact with the eyes
- •
- do not exceed recommended dosage
- •
- do not use for more than 7 days unless directed by a doctor/dentist
DIRECTIONS
Anbesol Regular Strength Cool Mint and Maximum Strength Liquid
- •
- adults and children 2 years of age and older:
- •
- wipe liquid on with cotton, or cotton swab, or fingertip
- •
- apply to the affected area up to 4 times daily or as directed by a doctor/dentist
- •
- children under 12 years of age: adult supervision should be given in the use of this product
- •
- children under 2 years of age: do not use
Anbesol Regular Strength Cool Mint and Maximum Strength Gel
- •
- to open tube, cut tip of the tube on score mark with scissors
- •
- adults and children 2 years of age and older: apply a pea-size amount to the affected area up to 4 times daily or as directed by a doctor/dentist
- •
- children under 12 years of age: adult supervision should be given in the use of this product
- •
- children under 2 years of age: do not use
- •
- for denture irritation:
- •
- apply thin layer to the affected area
- •
- do not reinsert dental work until irritation/pain is relieved
- •
- rinse mouth well before reinserting
OTHER INFORMATION
INACTIVE INGREDIENTS
Anbesol Regular Strength Cool Mint Liquid
benzyl alcohol, D&C red no. 33, D&C yellow no. 10, FD&C blue no. 1, FD&C yellow no. 6, methylparaben, natural and artificial flavors, polyethylene glycol, propylene glycol, saccharin
Anbesol Regular Strength Cool Mint Gel
benzyl alcohol, carbomer 934P, D&C red no. 33, D&C yellow no. 10, FD&C blue no. 1, FD&C yellow no. 6, glycerin, methylparaben, natural and artificial flavor, polyethylene glycol, propylene glycol, saccharin
PRODUCT PACKAGING
The product packaging shown below represents a sample of that currently in use. Additional packaging may also be available.
PRINCIPAL DISPLAY PANEL - 9 g Tube Blister Pack - 10%
cool mint
gel
See new warnings information
Anbesol®
Oral Anesthetic/Benzocaine 10%
REGULAR
STRENGTH
DOCTOR
RECOMMENDED
Instant
Pain Relief
- •
- Toothaches
- •
- Gum Pain
- •
- Canker Sores
- •
- Denture Pain
NET WT 0.33 OZ (9 g)
Safety Sealed Tube:
Do Not Use if tube tip is cut prior to opening.
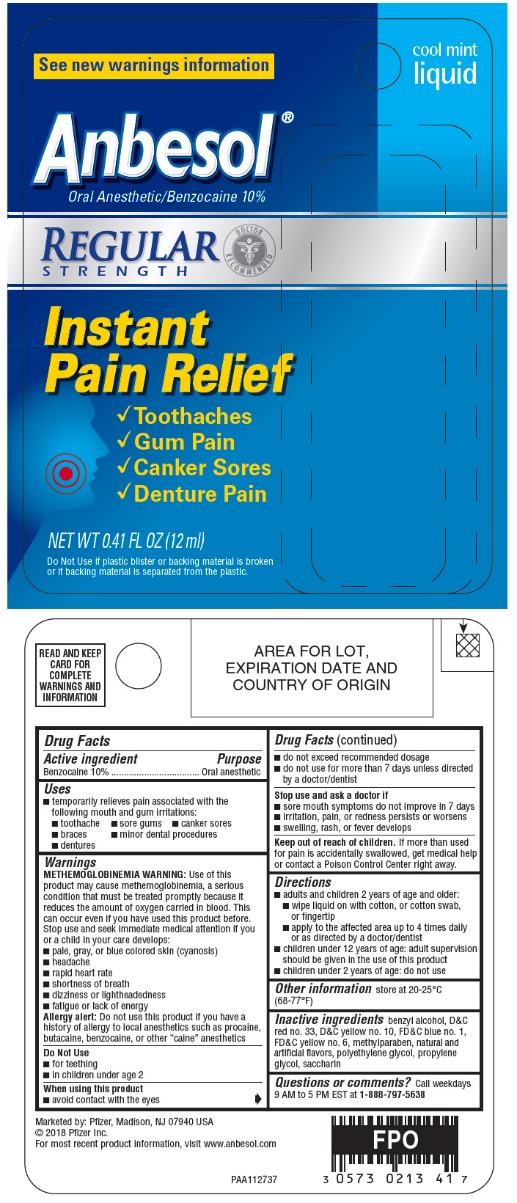
PRINCIPAL DISPLAY PANEL - 12 ml Bottle Blister Pack - 10%
cool mint
liquid
See new warnings information
Anbesol®
Oral Anesthetic/Benzocaine 10%
REGULAR
STRENGTH
DOCTOR
RECOMMENDED
Instant
Pain Relief
- •
- Toothaches
- •
- Gum Pain
- •
- Canker Sores
- •
- Denture Pain
NET WT 0.41 FL OZ (12 ml)
Do Not Use if plastic blister or backing material is broken
or if backing material is separated from the plastic.
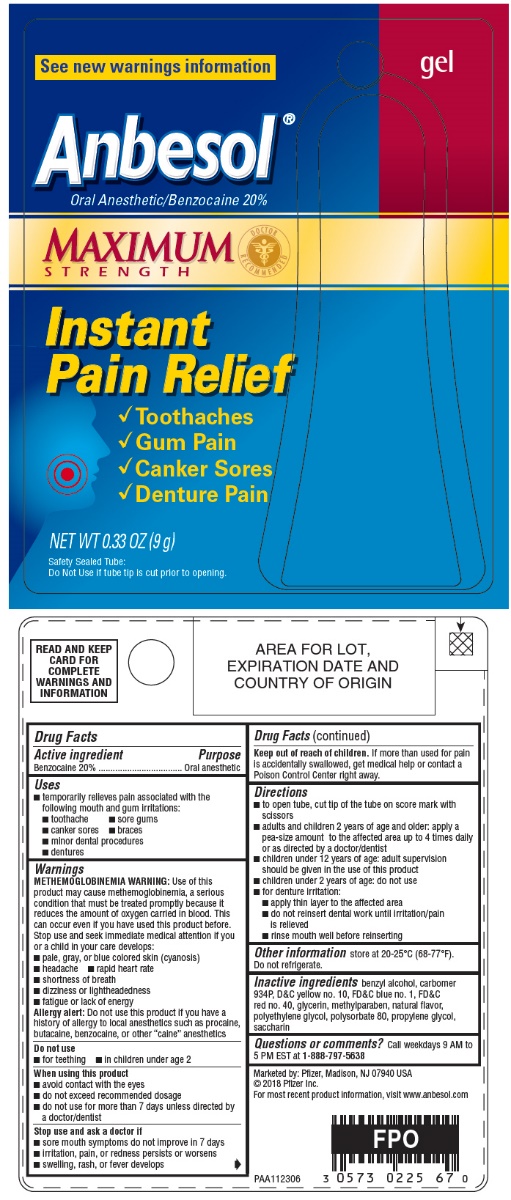
PRINCIPAL DISPLAY PANEL - 9 g Tube Blister Pack - 20%
gel
See new warnings information
Anbesol®
Oral Anesthetic/Benzocaine 20%
MAXIMUM
STRENGTH
DOCTOR
RECOMMENDED
Instant
Pain Relief
- •
- Toothaches
- •
- Gum Pain
- •
- Canker Sores
- •
- Denture Pain
NET WT 0.33 OZ (9 g)
Safety Sealed Tube:
Do Not Use if tube tip is cut prior to opening.
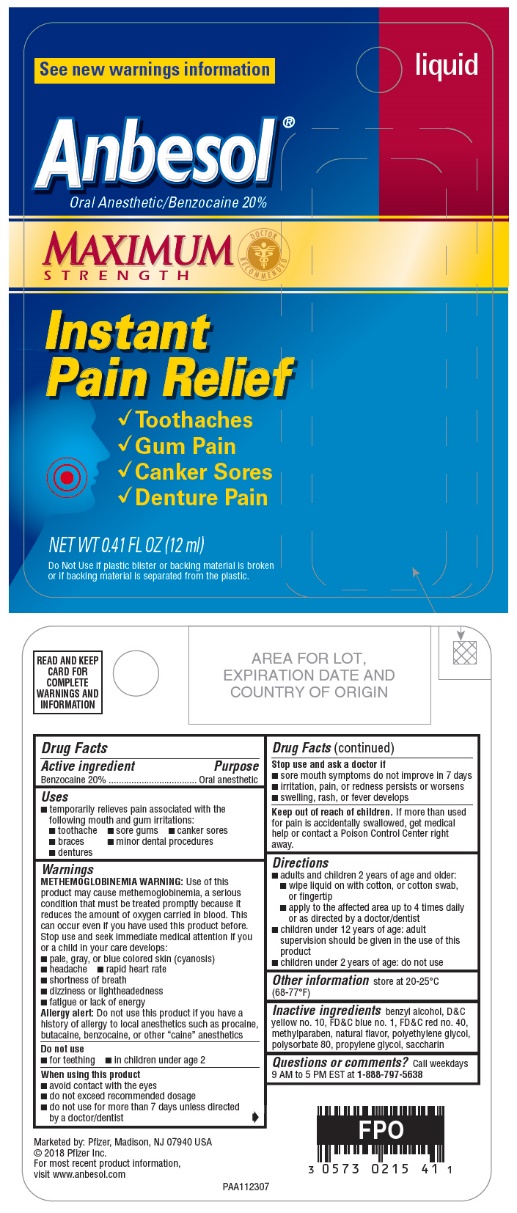
PRINCIPAL DISPLAY PANEL - 12 ml Bottle Blister Pack - 20%
liquid
See new warnings information
Anbesol®
Oral Anesthetic/Benzocaine 20%
MAXIMUM
STRENGTH
DOCTOR
RECOMMENDED
Instant
Pain Relief
- •
- Toothaches
- •
- Gum Pain
- •
- Canker Sores
- •
- Denture Pain
NET WT 0.41 FL OZ (12 ml)
Do Not Use if plastic blister or backing material is broken
or if backing material is separated from the plastic.
| ANBESOL REGULAR STRENGTH
benzocaine gel |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| ANBESOL REGULAR STRENGTH
benzocaine solution |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| ANBESOL MAXIMUM STRENGTH
benzocaine gel |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| ANBESOL MAXIMUM STRENGTH
benzocaine solution |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263) |