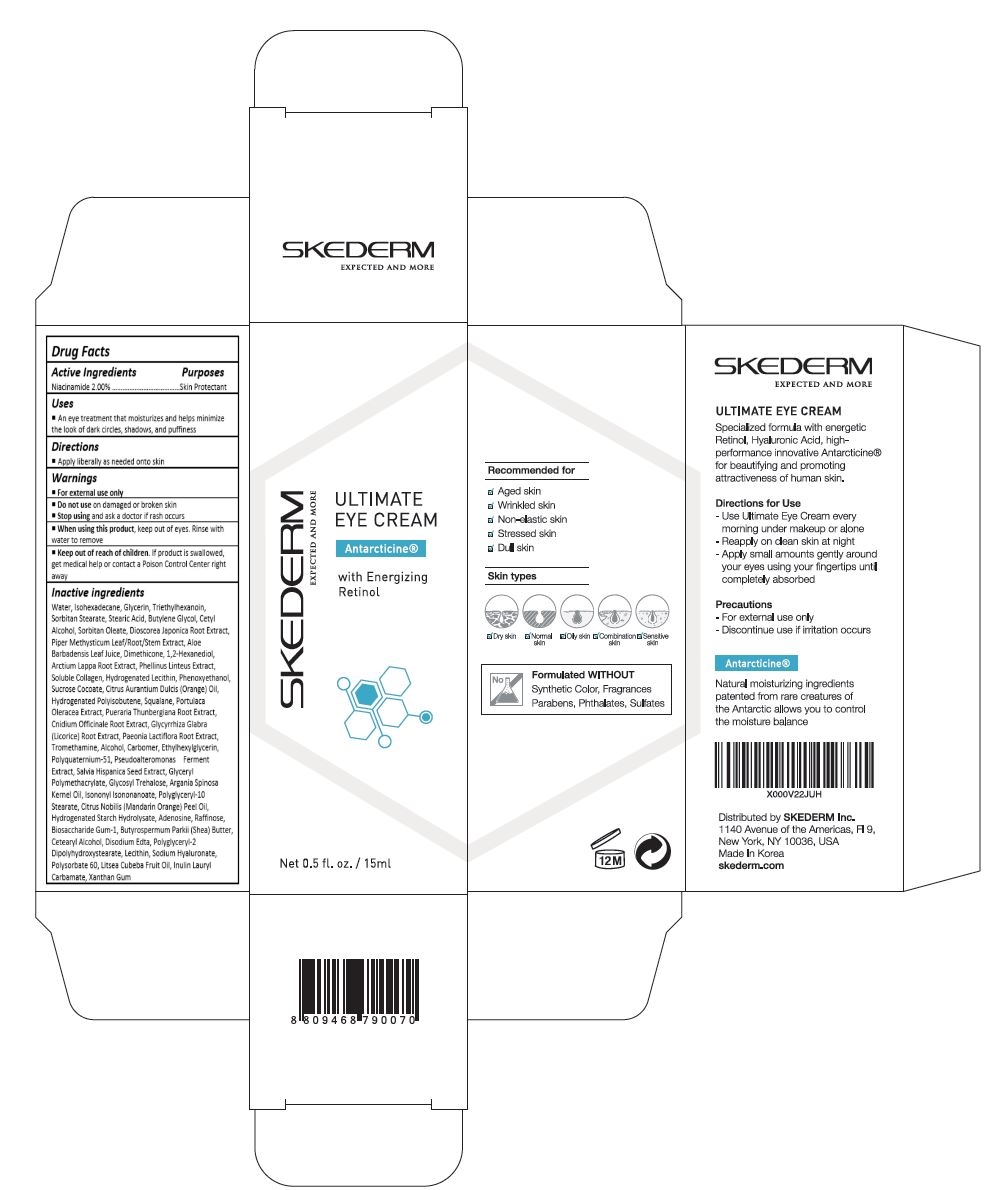
SKEDERM ULTIMATE EYE- niacinamide cream
Skederm Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
70209-001_Deactivation
An eye treatment that moisturizes and helps minimize the look of dark circles, shadows, and puffiness
For external use only.
Do not use on damaged or broken skin.
When using this product, keep out of eyes. Rinse with water to remove.
Stop using and ask a doctor if rash occurs.
Keep out of reach of the children. If product is swallowed, get medical help or contact a poison control center right away.
Water, Isohexadecane, Glycerin, Triethylhexanoin, Sorbitan Stearate, Stearic Acid, Butylene Glycol, Cetyl Alcohol, Sorbitan Oleate, Dioscorea Japonica Root Extract, Phellinus Linteus Extract, Arctium Lappa Root Extract, Piper Methysticum Leaf/Root/Stem Extract, Portulaca Oleracea Extract, Pueraria Thunbergiana Root Extract, Glycyrrhiza Glabra (Licorice) Root Extract, Paeonia Lactiflora Root Extract, Cnidium Officinale Root Extract, Pseudoalteromonas Ferment Extract, Salvia Hispanica Seed Extract, Citrus Aurantium Dulcis (Orange) Oil, Soluble Collagen, Hydrogenated Lecithin, Aloe Barbadensis Leaf Juice, Argania Spinosa Kernel Oil, Citrus Nobilis (Mandarin Orange) Peel Oil, Sodium Hyaluronate, Litsea Cubeba Fruit Oil, Dimethicone, 1,2-Hexanediol, Polysorbate 60, Sucrose Cocoate, Tromethamine, Squalane, Hydrogenated Polyisobutene, Ethylhexylglycerin, Alcohol, Carbomer, Polyquaternium-51, Glycosyl Trehalose, Glyceryl Polymethacrylate, Isononyl Isononanoate, Polyglyceryl-10 Stearate, Hydrogenated Starch Hydrolysate, Raffinose, Disodium EDTA, Biosaccharide Gum-1, Cetearyl Alcohol, Butyrospermum Parkii (Shea) Butter, Polyglyceryl-2 Dipolyhydroxystearate, Lecithin, Panthenol, Inulin Lauryl Carbamate, Xanthan Gum, Folic Acid, Disodium Stearoyl Glutamate, Hydrolyzed Hyaluronic Acid, Ceramide Np, Cholesterol, Caprylyl Glycol, Retinol, Palmitoyl Pentapeptide-4, Phenoxyethanol
| SKEDERM ULTIMATE EYE
niacinamide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Skederm Inc. (079981781) |
| Registrant - Skederm Inc. (079981781) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Kolmar Korea Co., Ltd. | 689512611 | manufacture(70209-001) | |