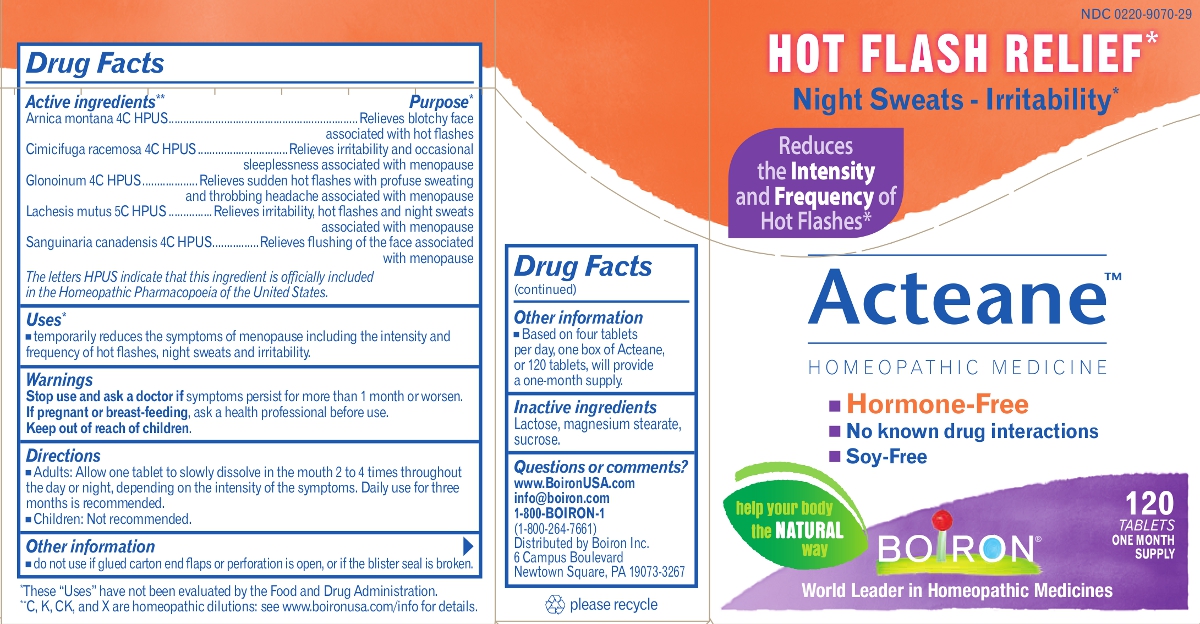
ACTEANE- arnica montana, black cohosh, nitroglycerin, lachesis muta venom, sanguinaria canadensis root tablet
Laboratoires Boiron
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Acteane
Active Ingredients
Arnica montana 4C HPUS
Cimicifuga racemosa 4C HPUS
Glonoinum 4C HPUS
Lachesis mutus 5C HPUS
Sanguinaria canadensis 4C HPUS
Purpose
| Arnica montana | ....... | Relieves blotchy face associated with hot flashes |
| Cimicifuga racemosa | ....... | Relieves irritability and occasional sleeplessness associated with menopause |
| Glonoinum | ....... | Relieves sudden hot flashes with profuse sweating and throbbing headache associated with menopause |
| Lachesis mutus | ....... | Relieves irritability, hot flashes and night sweats associated with menopause |
| Sanguinaria canadensis | ....... | Relieves flushing of the face associated with menopause |
Uses
• temporarily reduces the symptoms of menopause including the intensity and frequency of hot flashes, night sweats and irritability.
Directions
- Adults: Allow one tablet to slowly dissolve in the mouth 2 to 4 times throughout the day or night, depending on the intensity of the symptoms. Daily use for three months is recommended.
- Children: Not recommended.
Other information
- do not use if glued carton end flaps or perforation is open, or if the blister seal is broken.
- Based on four tablets per day, one box of Acteane, or 120 tablets, will provide a one-month supply.
| ACTEANE
arnica montana, black cohosh, nitroglycerin, lachesis muta venom, sanguinaria canadensis root tablet |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - Laboratoires Boiron (282560473) |
| Registrant - Boiron Inc. (014892269) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boiron | 282560473 | manufacture(0220-9070) | |
Revised: 11/2023
Document Id: 0b51328e-2a95-f0a1-e063-6294a90aedec
Set id: 91a01230-35cf-47ce-8f1c-452d152ddf3a
Version: 3
Effective Time: 20231129
Laboratoires Boiron