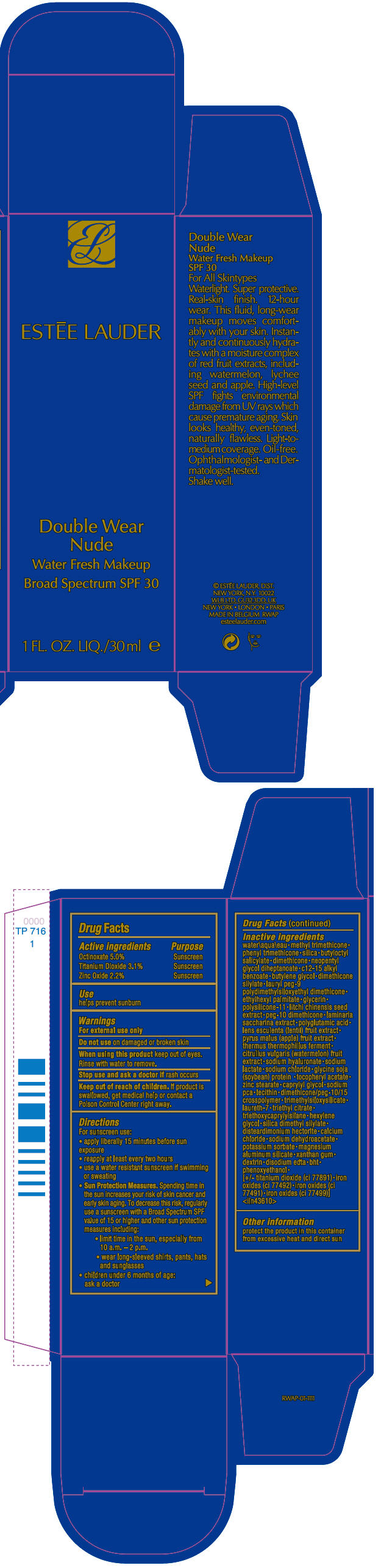
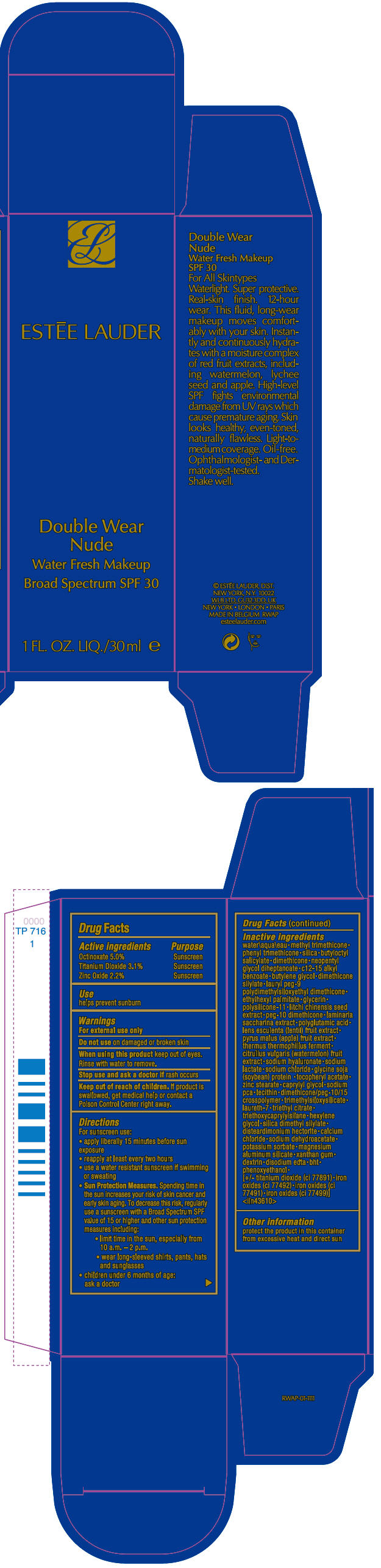
Label: DOUBLE WEAR NUDE WATER FRESH MAKEUP BROAD SPECTRUM SPF 30- octinoxate, titanium dioxide, and zinc oxide liquid
- NDC Code(s): 11559-048-01, 11559-048-02
- Packager: ESTEE LAUDER INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Use
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive ingredients
water\aqua\eau • methyl trimethicone • phenyl trimethicone • silica • butyloctyl salicylate • dimethicone • neopentyl glycol diheptanoate • c12-15 alkyl benzoate • butylene glycol • dimethicone silylate • lauryl peg-9 polydimethylsiloxyethyl dimethicone • ethylhexyl palmitate • glycerin • polysilicone-11 • litchi chinensis seed extract • peg-10 dimethicone • laminaria saccharina extract • polyglutamic acid • lens esculenta (lentil) fruit extract • pyrus malus (apple) fruit extract • thermus thermophillus ferment • citrullus vulgaris (watermelon) fruit extract • sodium hyaluronate • sodium lactate • sodium chloride • glycine soja (soybean) protein • tocopheryl acetate • zinc stearate • caprylyl glycol • sodium pca • lecithin • dimethicone/peg-10/15 crosspolymer • trimethylsiloxysilicate • laureth-7 • triethyl citrate • triethoxycaprylylsilane • hexylene glycol • silica dimethyl silylate • disteardimonium hectorite • calcium chloride • sodium dehydroacetate • potassium sorbate • magnesium aluminum silicate • xanthan gum • dextrin • disodium edta • bht • phenoxyethanol • [+/- titanium dioxide (ci 77891) • iron oxides (ci 77492) • iron oxides (ci 77491) • iron oxides (ci 77499)] <iln43610>
- Other information
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton
-
INGREDIENTS AND APPEARANCE
DOUBLE WEAR NUDE WATER FRESH MAKEUP BROAD SPECTRUM SPF 30
octinoxate, titanium dioxide, and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11559-048 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 50 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 31 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 22 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) METHYL TRIMETHICONE (UNII: S73ZQI0GXM) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) DIMETHICONE (UNII: 92RU3N3Y1O) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) ETHYLHEXYL PALMITATE (UNII: 2865993309) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) LITCHI CHINENSIS SEED (UNII: 9294024N9Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) LENS CULINARIS FRUIT (UNII: ZYZ076G9JH) APPLE (UNII: B423VGH5S9) THERMUS THERMOPHILUS LYSATE (UNII: 775R692494) WATERMELON (UNII: 231473QB6R) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM LACTATE (UNII: TU7HW0W0QT) SODIUM CHLORIDE (UNII: 451W47IQ8X) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ZINC STEARATE (UNII: H92E6QA4FV) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) LAURETH-7 (UNII: Z95S6G8201) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) HEXYLENE GLYCOL (UNII: KEH0A3F75J) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) XANTHAN GUM (UNII: TTV12P4NEE) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11559-048-01 1 in 1 CARTON 05/01/2017 07/15/2025 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:11559-048-02 1 in 1 CARTON 03/02/2022 12/31/2023 2 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/01/2017 07/15/2025 Labeler - ESTEE LAUDER INC (005914387) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations Estee Lauder N.V. 370151326 manufacture(11559-048) , pack(11559-048) , label(11559-048)