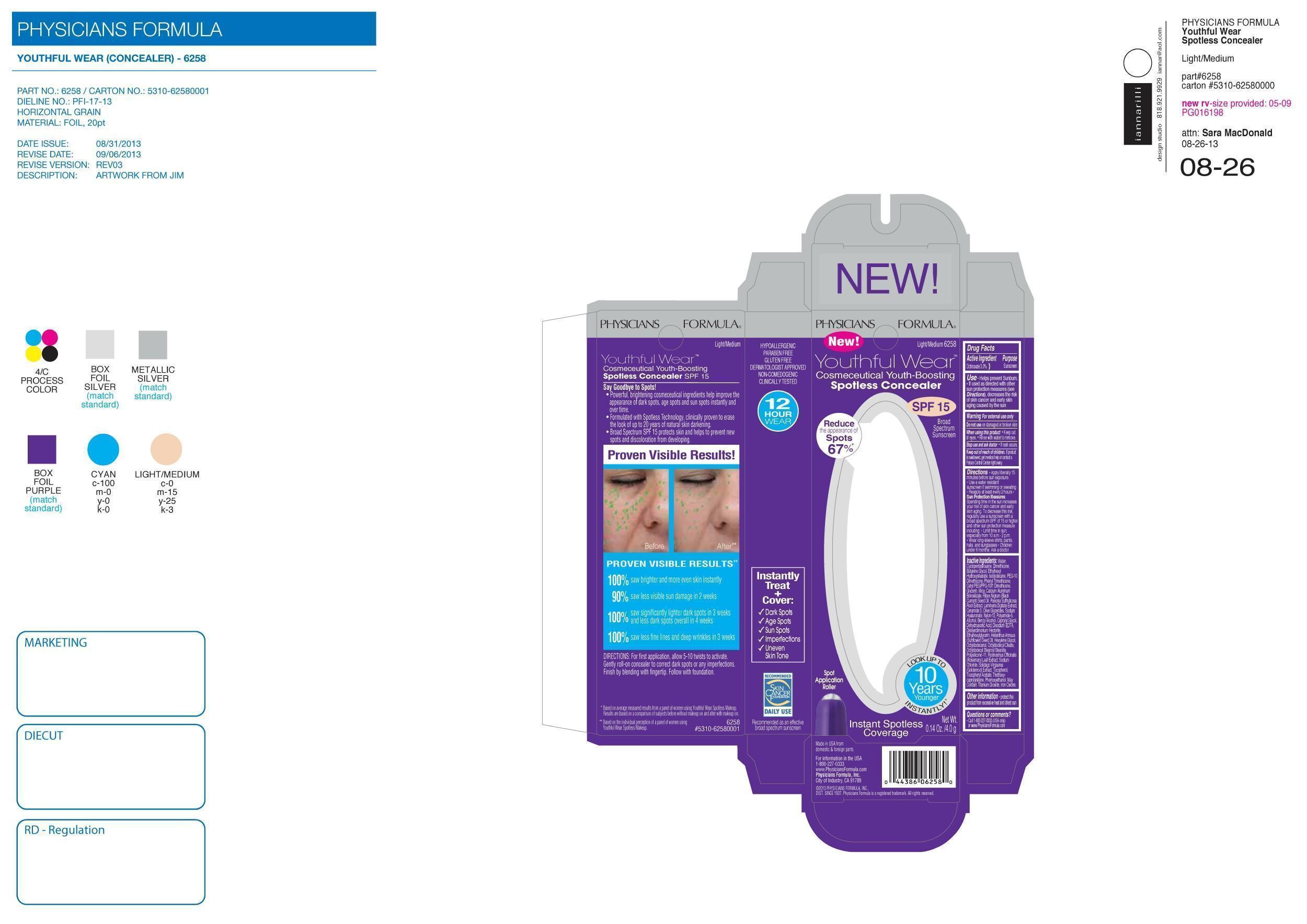
YOUTHFUL WEAR SPOTLESS CONCEALER SPF 15- octinoxate lotion
Physicians Formula Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
| YOUTHFUL WEAR SPOTLESS CONCEALER
SPF 15
octinoxate lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Physicians Formula Inc (021261805) |
| Registrant - Markwins International Corp. (113313969) |
Revised: 12/2018
Document Id: 7e0b31d5-f2e2-66cc-e053-2a91aa0a42ce
Set id: 8eaec5ef-d3e7-40d2-858d-22d758a87938
Version: 3
Effective Time: 20181227
Physicians Formula Inc