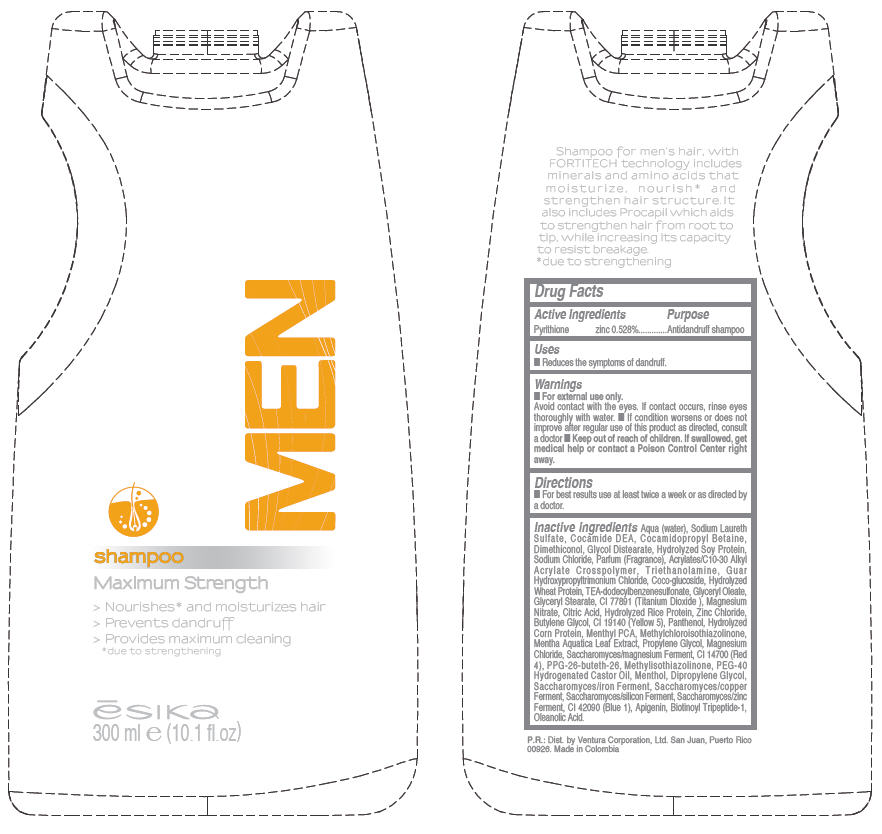
Label: ESIKA MEN MAXIMUM STRENGTH- pyrithione zinc liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 13537-154-01 - Packager: Ventura Corporation Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 27, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
WATER, SODIUM LAURETH SULFATE, COCAMIDE DEA, COCAMIDOPROPYL BETAINE, DIMETHICONOL, GLYCOL DISTEARATE, HYDROLYZED SOY PROTEIN, SODIUM CHLORIDE, FRAGRANCE, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, TRIETHANOLAMINE, GUAR HYDROXYPROPYLTRIMONIUM CHLORIDE, COCO-GLUCOSIDE, HYDROLYZED WHEAT PROTEIN, TEA-DODECYLBENZENESULFONATE, GLYCERYL OLEATE, GLYCERYL STEARATE, CI 77891 (TITANIUM DIOXIDE ), MAGNESIUM NITRATE, CITRIC ACID, HYDROLYZED RICE PROTEIN, ZINC CHLORIDE, BUTYLENE GLYCOL, CI 19140 (YELLOW 5), PANTHENOL, HYDROLYZED CORN PROTEIN, MENTHYL PCA, METHYLCHLOROISOTHIAZOLINONE, MENTHA AQUATICA LEAF EXTRACT, PROPYLENE GLYCOL, MAGNESIUM CHLORIDE, SACCHAROMYCES/MAGNESIUM FERMENT, CI 14700 (RED 4), PPG-26-BUTETH-26, METHYLISOTHIAZOLINONE, PEG-40 HYDROGENATED CASTOR OIL, MENTHOL, DIPROPYLENE GLYCOL, SACCHAROMYCES/IRON FERMENT, SACCHAROMYCES/COPPER FERMENT, SACCHAROMYCES/SILICON FERMENT, SACCHAROMYCES/ZINC FERMENT, CI 42090 (BLUE 1), APIGENIN, BIOTINOYL TRIPEPTIDE-1, OLEANOLIC ACID
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 300 ml Bottle
-
INGREDIENTS AND APPEARANCE
ESIKA MEN MAXIMUM STRENGTH
pyrithione zinc liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-154 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC 0.00528 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) COCO DIETHANOLAMIDE (UNII: 92005F972D) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) GLYCOL DISTEARATE (UNII: 13W7MDN21W) SODIUM CHLORIDE (UNII: 451W47IQ8X) TROLAMINE (UNII: 9O3K93S3TK) COCO GLUCOSIDE (UNII: ICS790225B) TEA-DODECYLBENZENESULFONATE (UNII: 8HM7ZD48HN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MAGNESIUM NITRATE (UNII: 77CBG3UN78) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ZINC CHLORIDE (UNII: 86Q357L16B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) PANTHENOL (UNII: WV9CM0O67Z) MENTHYL PYRROLIDONE CARBOXYLATE, (-),DL- (UNII: 8P18J856U2) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) MENTHA AQUATICA LEAF (UNII: 5M106ME6PT) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) FD&C RED NO. 4 (UNII: X3W0AM1JLX) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) MENTHOL (UNII: L7T10EIP3A) DIPROPYLENE GLYCOL (UNII: E107L85C40) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) APIGENIN (UNII: 7V515PI7F6) BIOTINOYL TRIPEPTIDE-1 (UNII: O6380721VA) OLEANOLIC ACID (UNII: 6SMK8R7TGJ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-154-01 300 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part358H 01/24/2012 Labeler - Ventura Corporation Ltd (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE(13537-154)