BUSPIRONE HYDROCHLORIDE- buspirone hydrochloride tablet
REMEDYREPACK INC.
----------
DESCRIPTION
Buspirone hydrochloride tablets USP are an antianxiety agent that is not chemically or pharmacologically related to the benzodiazepines, barbiturates, or other sedative/anxiolytic drugs.
Buspirone hydrochloride is a white crystalline, water soluble compound. Chemically, buspirone hydrochloride is N-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-1,1-cyclopentanediacetamide monohydrochloride, which can be represented by the following structural formula:
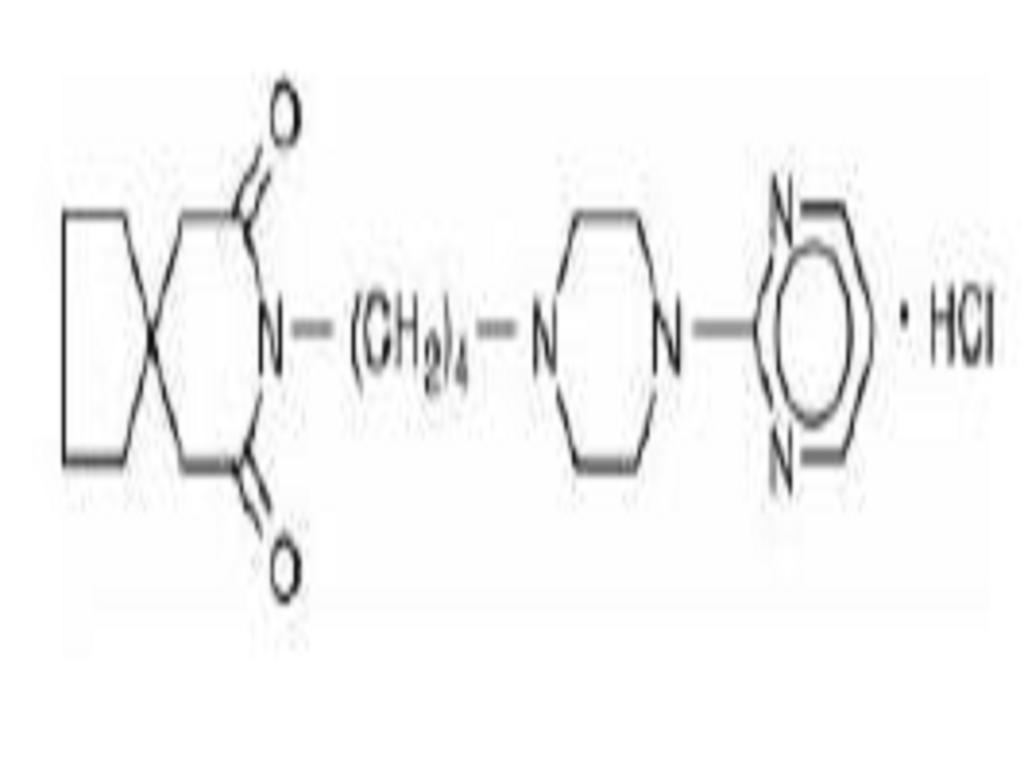
C21H31N5O2HCl M.W. 421.96
Each tablet, for oral administration, contains 5 mg, 10 mg, 15 mg or 30 mg of buspirone hydrochloride USP (equivalent to 4.6 mg, 9.1 mg, 13.7 mg and 27.4 mg of buspirone free base, respectively). The 5 mg and 10 mg tablets are scored so they can be bisected. Thus, the 5 mg tablet can also provide a 2.5 mg dose, and the 10 mg tablet can provide a 5 mg dose. The 15 mg tablets are scored such that they may be bisected or trisected. Thus, a single tablet can provide the following doses: 15 mg (entire tablet), 10 mg (two-thirds of a tablet), 7.5 mg (one-half of a tablet), or 5 mg (one-third of a tablet). The 30 mg tablets are scored such that they may be bisected or trisected. Thus, a single tablet can provide the following doses: 30 mg (entire tablet), 20 mg (two-thirds of a tablet), 15 mg (one-half of a tablet), or 10 mg (one-third of a tablet). Buspirone hydrochloride tablets USP contain the following inactive ingredients: anhydrous lactose, colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate.
CLINICAL PHARMACOLOGY
The mechanism of action of buspirone is unknown. Buspirone differs from typical benzodiazepine anxiolytics in that it does not exert anticonvulsant or muscle relaxant effects. It also lacks the prominent sedative effect that is associated with more typical anxiolytics. In vitro preclinical studies have shown that buspirone has a high affinity for serotonin (5-HT1A) receptors. Buspirone has no significant affinity for benzodiazepine receptors and does not affect GABA binding in vitro or in vivo when tested in preclinical models.
Buspirone has moderate affinity for brain D2-dopamine receptors. Some studies do suggest that buspirone may have indirect effects on other neurotransmitter systems.
Buspirone hydrochloride tablets are rapidly absorbed in man and undergo extensive first-pass metabolism. In a radiolabeled study, unchanged buspirone in the plasma accounted for only about 1% of the radioactivity in the plasma. Following oral administration, plasma concentrations of unchanged buspirone are very low and variable between subjects. Peak plasma levels of 1 ng/mL to 6 ng/mL have been observed 40 to 90 minutes after single oral doses of 20 mg. The single-dose bioavailability of unchanged buspirone when taken as a tablet is on the average about 90% of an equivalent dose of solution, but there is large variability.
The effects of food upon the bioavailability of buspirone hydrochloride tablets have been studied in eight subjects. They were given a 20 mg dose with and without food; the area under the plasma concentration-time curve (AUC) and peak plasma concentration (Cmax) of unchanged buspirone increased by 84% and 116%, respectively, but the total amount of buspirone immunoreactive material did not change. This suggests that food may decrease the extent of presystemic clearance of buspirone (see DOSAGE AND ADMINISTRATION).
A multiple-dose study conducted in 15 subjects suggests that buspirone has nonlinear pharmacokinetics. Thus, dose increases and repeated dosing may lead to somewhat higher blood levels of unchanged buspirone than would be predicted from results of single-dose studies.
An in vitro protein binding study indicated that approximately 86% of buspirone is bound to plasma proteins. It was also observed that aspirin increased the plasma levels of free buspirone by 23%, while flurazepam decreased the plasma levels of free buspirone by 20%. However, it is not known whether these drugs cause similar effects on plasma levels of free buspirone in vivo, or whether such changes, if they do occur, cause clinically significant differences in treatment outcome. An in vitro study indicated that buspirone did not displace highly protein-bound drugs such as phenytoin, warfarin, and propranolol from plasma protein, and that buspirone may displace digoxin.
Buspirone is metabolized primarily by oxidation, which in vitro has been shown to be mediated by cytochrome P450 3A4 (CYP3A4) (see PRECAUTIONS, Drug Interactions). Several hydroxylated derivatives and a pharmacologically active metabolite, 1-pyrimidinylpiperazine (1-PP), are produced. In animal models predictive of anxiolytic potential, 1-PP has about one quarter of the activity of buspirone, but is present in up to 20 fold greater amounts. However, this is probably not important in humans: blood samples from humans chronically exposed to buspirone hydrochloride tablets do not exhibit high levels of 1-PP; mean values are approximately 3 ng/mL and the highest human blood level recorded among 108 chronically dosed patients was 17 ng/mL, less than 1/200th of 1-PP levels found in animals given large doses of buspirone without signs of toxicity.
In a single-dose study using 14C-labeled buspirone, 29% to 63% of the dose was excreted in the urine within 24 hours, primarily as metabolites; fecal excretion accounted for 18% to 38% of the dose. The average elimination half-life of unchanged buspirone after single doses of 10 to 40 mg is about 2 to 3 hours.
INDICATIONS & USAGE
Buspirone hydrochloride tablets are indicated for the management of anxiety disorders or the short-term relief of the symptoms of anxiety. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
The efficacy of buspirone hydrochloride tablets has been demonstrated in controlled clinical trials of outpatients whose diagnosis roughly corresponds to Generalized Anxiety Disorder (GAD). Many of the patients enrolled in these studies also had coexisting depressive symptoms and buspirone hydrochloride tablets relieved anxiety in the presence of these coexisting depressive symptoms. The patients evaluated in these studies had experienced symptoms for periods of 1 month to over 1 year prior to the study, with an average symptom duration of 6 months. Generalized Anxiety Disorder (300.02) is described in the American Psychiatric Association's Diagnostic and Statistical Manual, III1 as follows:
Generalized, persistent anxiety (of at least 1 month continual duration), manifested by symptoms from three of the four following categories:
1. Motor tension: shakiness, jitteriness, jumpiness, trembling, tension, muscle aches, fatigability, inability to relax, eyelid twitch, furrowed brow, strained face, fidgeting, restlessness, easy startle.
2. Autonomic hyperactivity: sweating, heart pounding or racing, cold, clammy hands, dry mouth, dizziness, lightheadedness, paresthesias (tingling in hands or feet), upset stomach, hot or cold spells, frequent urination, diarrhea, discomfort in the pit of the stomach, lump in the throat, flushing, pallor, high resting pulse and respiration rate.
3. Apprehensive expectation: anxiety, worry, fear, rumination, and anticipation of misfortune to self or others.
4. Vigilance and scanning: hyperattentiveness resulting in distractibility, difficulty in concentrating, insomnia, feelingon edge,irritability, impatience.
The above symptoms would not be due to another mental disorder, such as a depressive disorder or schizophrenia. However, mild depressive symptoms are common in GAD.
The effectiveness of buspirone hydrochloride tablets in long-term use, that is, for more than 3 to 4 weeks, has not been demonstrated in controlled trials. There is no body of evidence available that systematically addresses the appropriate duration of treatment for GAD. However, in a study of long-term use, 264 patients were treated with buspirone hydrochloride tablets for 1 year without ill effect. Therefore, the physician who elects to use buspirone hydrochloride tablets for extended periods should periodically reassess the usefulness of the drug for the individual patient.
CONTRAINDICATIONS
Buspirone hydrochloride tablets are contraindicated in patients hypersensitive to buspirone hydrochloride.
WARNINGS
The administration of buspirone hydrochloride tablets to a patient taking a monoamine oxidase inhibitor (MAOI) may pose a hazard
. There have been reports of the occurrence of elevated blood pressure when buspirone hydrochloride has been added to a regimen including an MAOI. Therefore, it is recommended that buspirone hydrochloride tablets not be used concomitantly with an MAOI.
Because buspirone hydrochloride tablets has no established antipsychotic activity, it should not be employed in lieu of appropriate antipsychotic treatment.
INFORMATION FOR PATIENTS
To assure safe and effective use of buspirone hydrochloride tablets, the following information and instructions should be given to patients:
1. Inform your physician about any medications, prescription or non-prescription, alcohol, or drugs that you are now taking or plan to take during your treatment with buspirone hydrochloride tablets.
2. Inform your physician if you are pregnant, or if you are planning to become pregnant, or if you become pregnant while you are taking buspirone hydrochloride tablets.
3. Inform your physician if you are breastfeeding an infant.
4. Until you experience how this medication affects you, do not drive a car or operate potentially dangerous machinery.
5. You should take buspirone hydrochloride tablets consistently, either always with or always without food.
6. During your treatment with buspirone hydrochloride tablets, avoid drinking large amounts of grapefruit juice.
DRUG & OR LABORATORY TEST INTERACTIONS
Buspirone hydrochloride may interfere with the urinary metanephrine/catecholamine assay. It has been mistakenly read as metanephrine during routine assay testing for pheochromocytoma, resulting in a false positive laboratory result. Buspirone hydrochloride should therefore be discontinued for at least 48 hours prior to undergoing a urine collection for catecholamines.
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
With or without metabolic activation, buspirone did not induce point mutations in five strains of Salmonella typhimurium (Ames Test) or mouse lymphoma L5178YTK+ cell cultures, nor was DNA damage observed with buspirone in Wi-38 human cells. Chromosomal aberrations or abnormalities did not occur in bone marrow cells of mice given one or five daily doses of buspirone.
PREGNANCY
Teratogenic Effects
Pregnancy Category B
No fertility impairment or fetal damage was observed in reproduction studies performed in rats and rabbits at buspirone doses of approximately 30 times the maximum recommended human dose. In humans, however, adequate and well-controlled studies during pregnancy have not been performed. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
LABOR & DELIVERY
The effect of buspirone hydrochloride tablets on labor and delivery in women is unknown. No adverse effects were noted in reproduction studies in rats.
NURSING MOTHERS
The extent of the excretion in human milk of buspirone or its metabolites is not known. In rats, however, buspirone and its metabolites are excreted in milk. Buspirone hydrochloride tablets administration to nursing women should be avoided if clinically possible.
PEDIATRIC USE
The safety and effectiveness of buspirone were evaluated in two placebo-controlled 6 week trials involving a total of 559 pediatric patients (ranging from 6 to 17 years of age) with GAD. Doses studied were 7.5 mg to 30 mg b.i.d. (15 to 60 mg/day). There were no significant differences between buspirone and placebo with regard to the symptoms of GAD following doses recommended for the treatment of GAD in adults. Pharmacokinetic studies have shown that, for identical doses, plasma exposure to buspirone and its active metabolite, 1-PP, are equal to or higher in pediatric patients than adults. No unexpected safety findings were associated with buspirone in these trials. There are no long-term safety or efficacy data in this population.
DOSAGE & ADMINISTRATION
The recommended initial dose is 15 mg daily (7.5 mg b.i.d.). To achieve an optimal therapeutic response, at intervals of 2 to 3 days the dosage may be increased 5 mg per day, as needed. The maximum daily dosage should not exceed 60 mg per day. In clinical trials allowing dose titration, divided doses of 20 to 30 mg per day were commonly employed.
The bioavailability of buspirone is increased when given with food as compared to the fasted state (see CLINICAL PHARMACOLOGY). Consequently, patients should take buspirone in a consistent manner with regard to the timing of dosing; either always with or always without food.
When buspirone is to be given with a potent inhibitor of CYP3A4, the dosage recommendations described in the PRECAUTIONS, Drug Interactions section should be followed.
HOW SUPPLIED
BusPIRone Hydrochloride Tablets USP, 5 mg are available as white to off-white, round, beveled-edge tablets, debossedTVand53on one side and scored on the other side, packaged in bottles of 100 and 500 tablets.
BusPIRone Hydrochloride Tablets USP, 10 mg are available as white to off-white, round, beveled-edge tablets, debossedTEVAon one side and scored and debossed54on the other side, packaged in bottles of 100 and 500 tablets.
BusPIRone Hydrochloride Tablets USP, 15 mg are available as white to off-white, rectangular, tablets that can either be bisected or trisected, debossedTVand1003on bisect segments, and debossed5on each trisect segment, and packaged in bottles of 100 and 500 tablets.
BusPIRone Hydrochloride tablets USP, 30 mg are available as white to off-white, rectangular, tablets that can either be bisected or trisected, debossedTVand5200on bisect segments, and debossed10on each trisect segment, and packaged in bottles of 60 and 500 tablets.
STORAGE AND HANDLING
Store at 20to 25(68to 77[See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
REFERENCES
1. American Psychiatric Association, Ed.: Diagnostic and Statistical Manual of Mental DisordersIII, American Psychiatric Association, May 1980.
INFORMATION FOR PATIENTS
BUSPIRONE HYDROCHLORIDE TABLETS USP
Rx only
HOW TO USE:
BUSPIRONE HYDROCHLORIDE TABLETS, 15 mg
Response to buspirone varies among individuals. Your physician may find it necessary to adjust your dosage to obtain the proper response.
This tablet design makes dosage adjustments easy. Each tablet is scored and can be broken accurately to provide any of the following dosages: 15mg, the entire tablet; 10 mg, two-thirds of a tablet; 7.5mg, one-half of a tablet; 5 mg, one-third of a tablet.
To break a tablet accurately and easily, hold the tablet between your thumbs and index fingers close to the appropriate tablet score (groove) as shown below. Then, with the tablet score facing you, apply pressure and snap the tablet segments apart (segments breaking incorrectly should not be used).
| BUSPIRONE HYDROCHLORIDE
buspirone hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - REMEDYREPACK INC. (829572556) |
