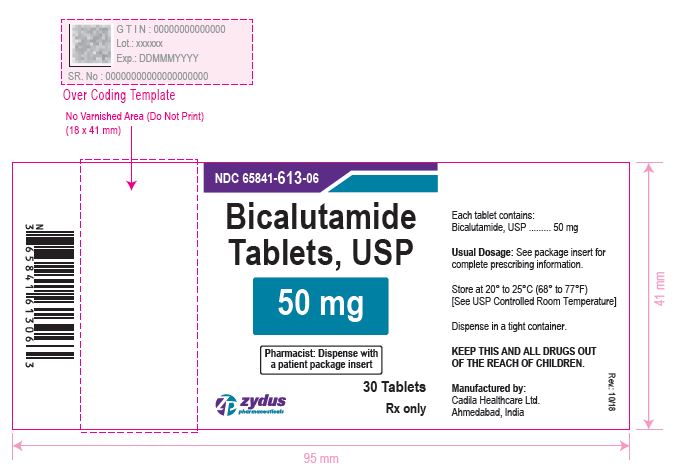
BICALUTAMIDE - bicalutamide tablet, film coated
Zydus Lifesciences Limited
----------
BICALUTAMIDE TABLETS
| BICALUTAMIDE
bicalutamide tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Zydus Lifesciences Limited (918596198) |
| Registrant - Zydus Lifesciences Limited (918596198) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Zydus Lifesciences Limited | 918596198 | ANALYSIS(65841-613) , MANUFACTURE(65841-613) | |
Revised: 12/2022
Document Id: 2b9f302e-27a7-4b8c-a678-dfe131ae0b90
Set id: 8e26fbf0-511c-44d9-b484-372d7b50b55e
Version: 8
Effective Time: 20221216
Zydus Lifesciences Limited