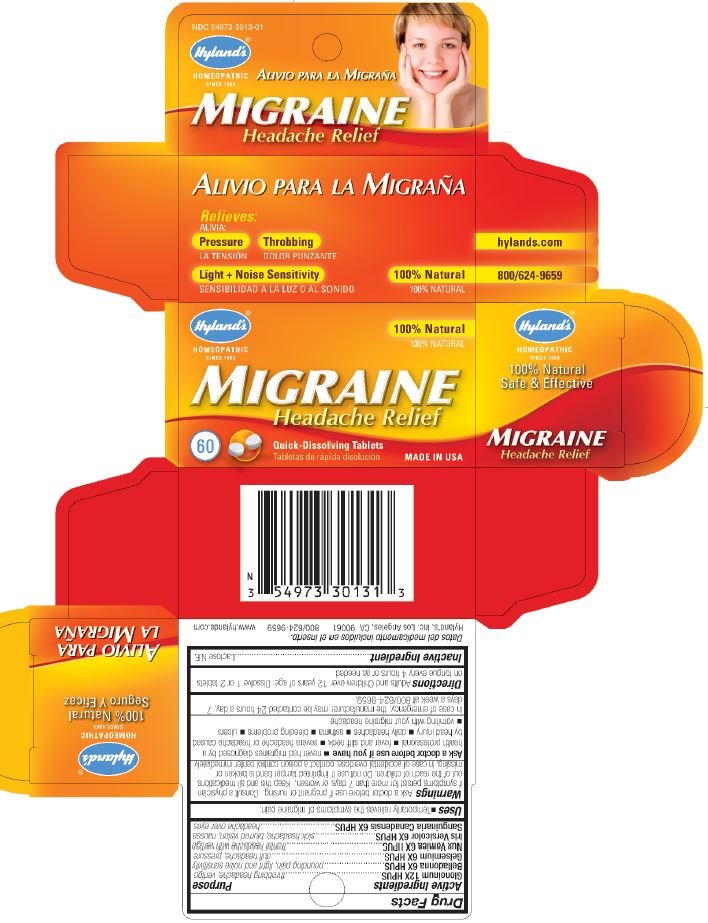
MIGRAINE HEADACHE RELIEF- nitroglycerin, atropa belladonna, gelsemium sempervirens root, strychnos nux-vomica seed, iris versicolor root, and sanguinaria canadensis root tablet, soluble
Hyland's
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Migraine Headache Relief
| Active Ingredients | Purpose |
| Glonoinum 12X HPUS | throbbing headache, vertigo |
| Belladonna 6X HPUS | pounding pain, light and noise sensitivity |
| Gelsemium 6X HPUS | dull headache, pressure |
| Nux Vomica 6X HPUS | frontal headache with vertigo |
| Iris Versicolor 6X HPUS | sick headache, blurred vision, nausea |
| Sanguinaria Canadensis 6X HPUS | headache over eyes |
Warnings
Ask a doctor before use if pregnant or nursing. Consult a physician if symptoms persist for more than 7 days or worsen.
In case of emergency, the manufacturer may be contacted 24 hours a day, 7 days a week at 800/624-9659.
| MIGRAINE HEADACHE RELIEF
nitroglycerin, atropa belladonna, gelsemium sempervirens root, strychnos nux-vomica seed, iris versicolor root, and sanguinaria canadensis root tablet, soluble |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Hyland's (028570695) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Standard Homeopathic Company | 008316655 | manufacture(54973-3013) | |
Revised: 12/2021
Document Id: d44edb00-e1fd-2f20-e053-2a95a90a83f0
Set id: 8de56ff0-1ad6-435e-abac-20075808fa87
Version: 3
Effective Time: 20211229
Hyland's