ELIXICURE ALL NATURAL LAVENDER- menthol, camphor cream
Honest Globe Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
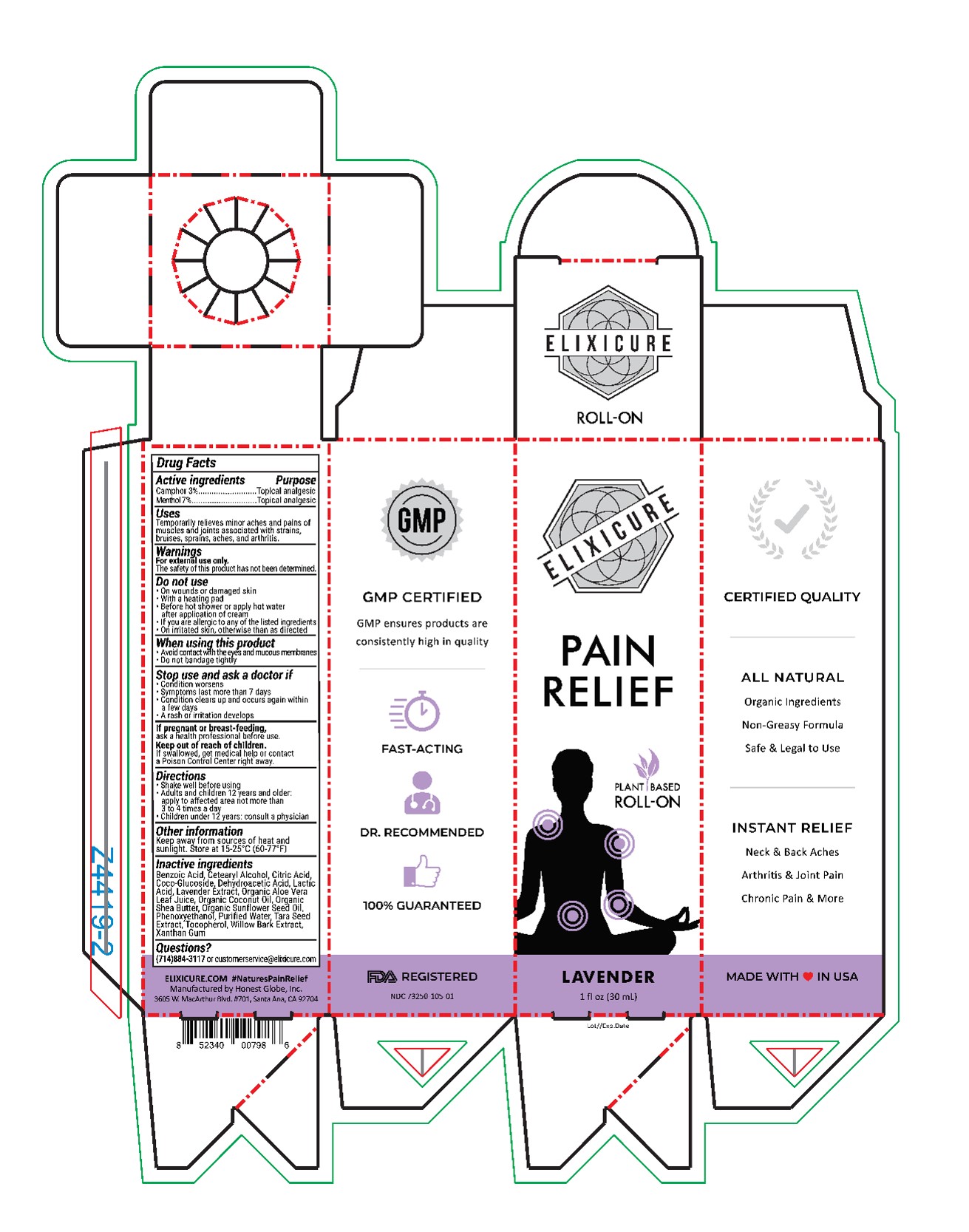
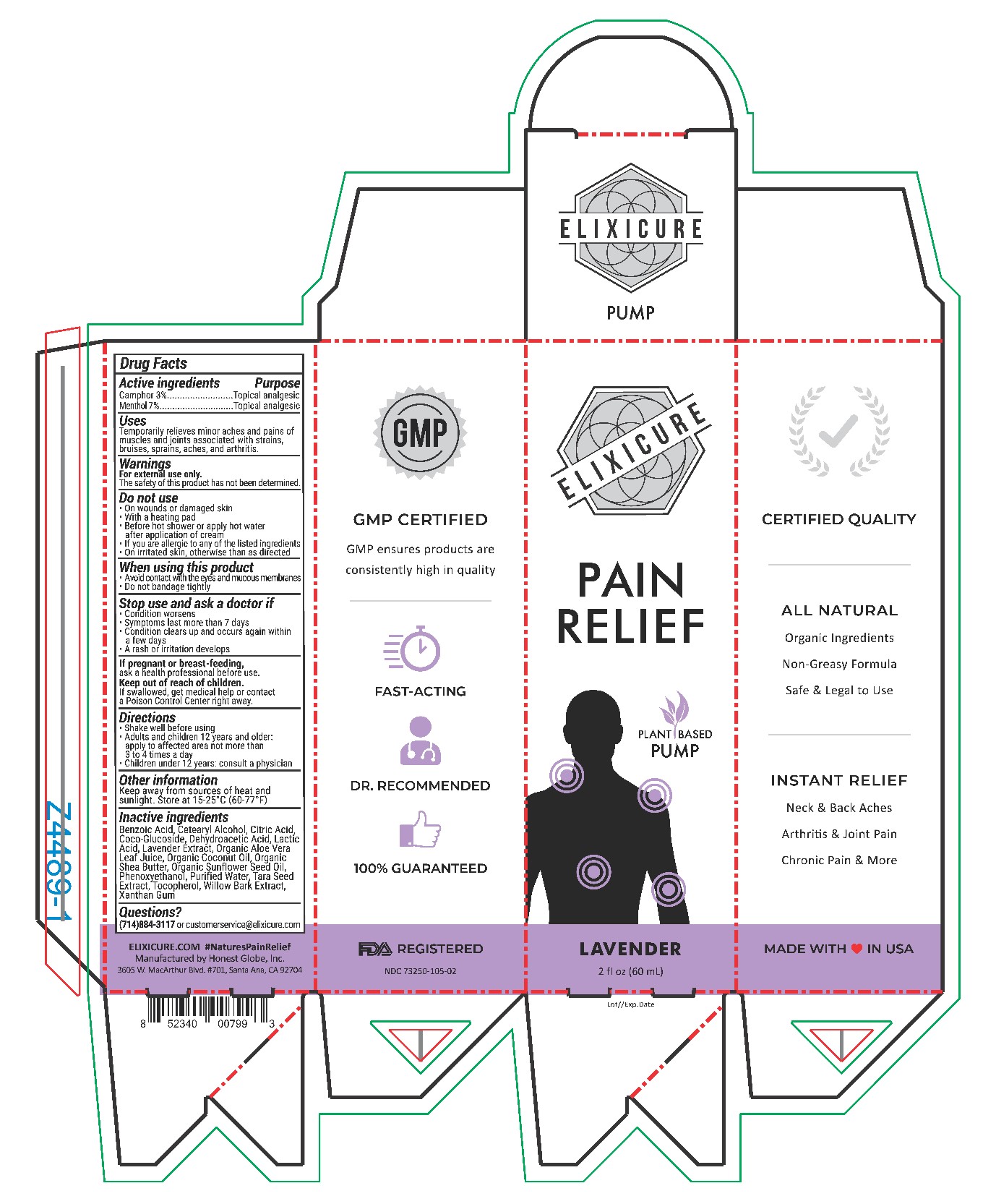
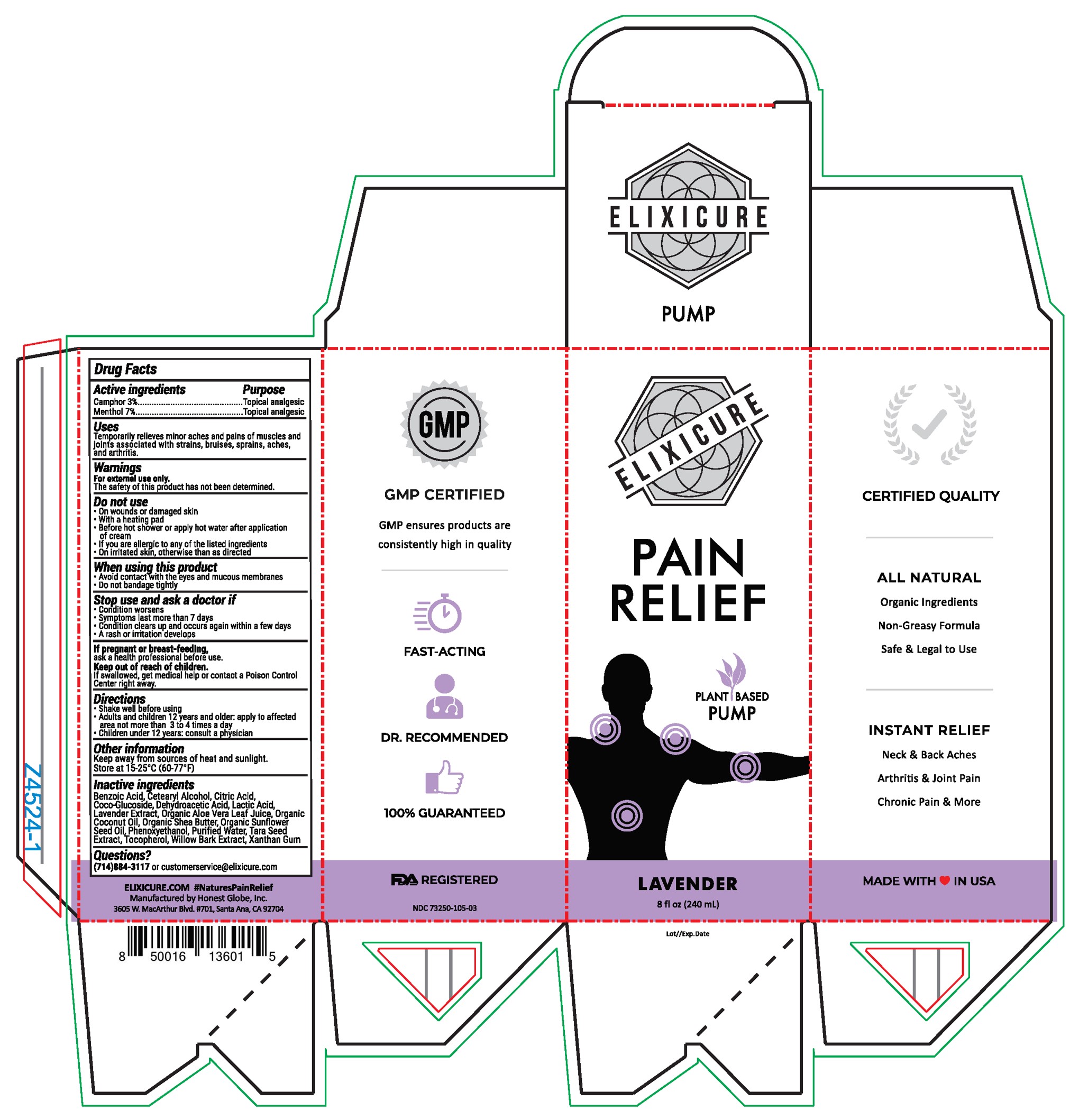
Elixicure All Natural Lavender
Uses
Temporarily relieves minor aches and pains of muscles and joints associated with strains, bruises, sprains, aches, and arthritis.
Do Not Use
On wounds or damaged skin
With a heating pad
Before hot shower or apply hot water after application of cream
If you are allergic to any of the listed ingredients
On irritated skin, otherwise than as directed
Stop Use and ask a doctor if
Condition worsens
Symptoms last more than 7 days
Condition clears up and occurs again with a few days
A rash or irritation develops
Keep Out Of Reach Of Children
If swallowed, get medical help or contact a Poison Control Center right away
Directions
Shake well before using
Adults and children 12 years or older: apply to affected area not more than 3-4 times a day
Children under age 12: Consult a physician
Inactive Ingredients
Purified Water, Organic Coconut Oil, Organic Sunflower Seed Oil, Cetearyl Alcohol, Coco-Glucoside, Organic Shea Butter, Xanthan Gum, Apricot Kernel Oil, Phenoxyethanol, Organic Aloe Vera Juice, Citric Acid, Tocopherol, Lactic Acid, Benzoic Acid, Dehydroacetic Acid, Wintergreen Extract, Willow Bark Extract, Tara Seed Extract
| ELIXICURE ALL NATURAL LAVENDER
menthol, camphor cream |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Honest Globe Inc (051811972) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Honest Globe Inc | 023554010 | manufacture(73250-105) | |