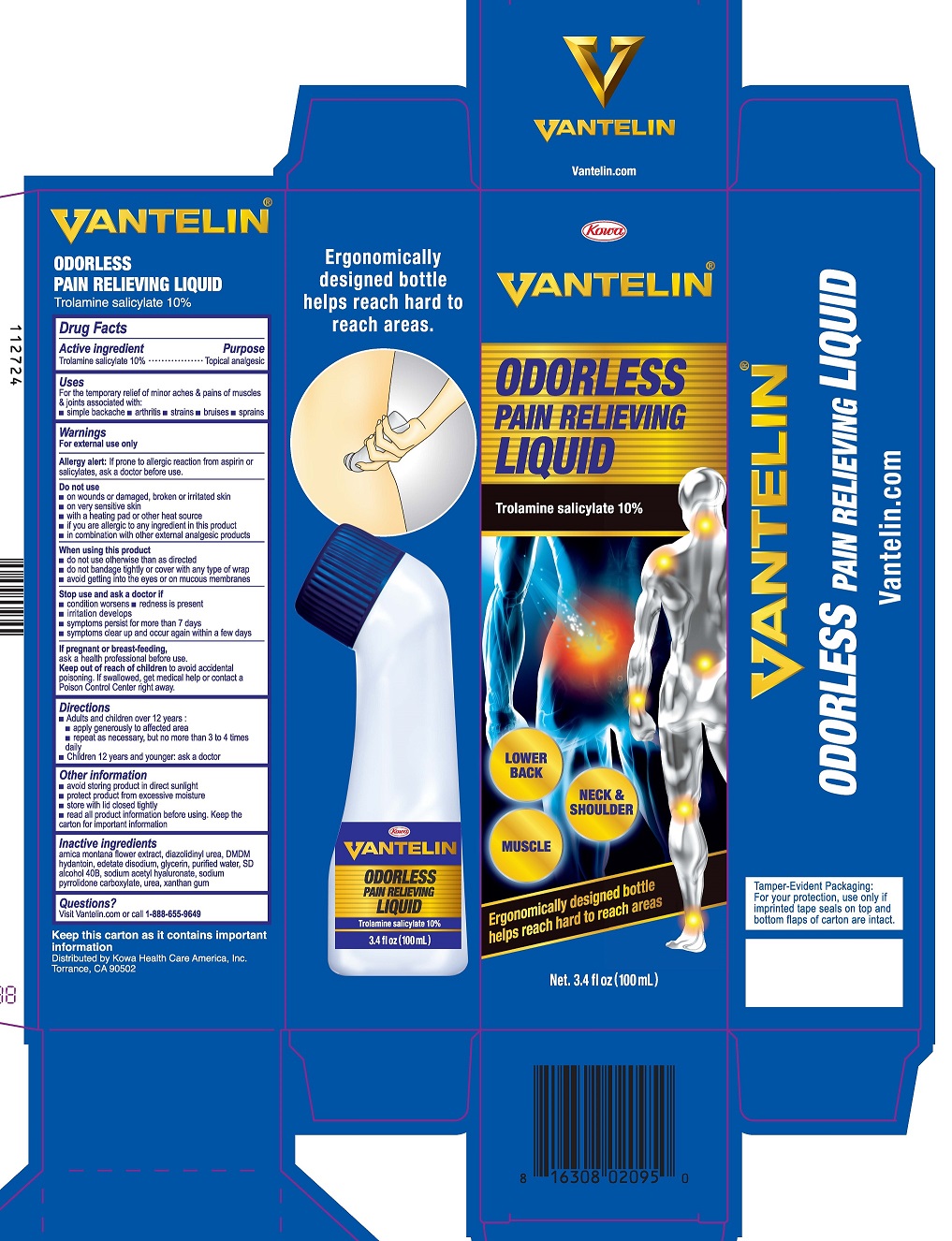
VANTELIN ODORLESS PAIN RELIEVING- trolamine salicylate liquid
Kowa Health Care America, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
VANTELIN ODORLESS PAIN RELIEVING LIQUID
Uses
For the temporary relief of minor aches & pains of muscles & joints associated with:
- simple backache
- arthritis
- strains
- bruises
- sprains
Warnings
For external use only
Allergy alert: If prone to allergic reaction from aspirin or salicylates, ask a doctor before use.
Do not use
- on wounds or damaged, broken or irritated skin
- on very sensitive skin
- with a heating pad or other heat source
- if you are allergic to any ingredient in this product
- in combination with other external analgesic products
When using this product
- do not use otherwise than as directed
- do not bandage tightly or cover with any type of wrap
- avoid getting into the eyes or on mucous membranes
Directions
- Adults and children over 12 years:
• apply generously to affected area
• repeat as necessary, but no more than 3 to 4 times daily - Children 12 years and younger: ask a doctor
Other information
- avoid storing product in direct sunlight
- protect product from excessive moisture
- store with lid closed tightly
- read all product information before using. Keep the carton for important information
| VANTELIN ODORLESS PAIN RELIEVING
trolamine salicylate liquid |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Kowa Health Care America, Inc. (078740352) |
Revised: 5/2018
Document Id: cde2eace-5f0d-4496-bff4-c0f48f6e88ec
Set id: 8c7ccf54-3e14-4dbb-a5dd-b93a724d0745
Version: 2
Effective Time: 20180509
Kowa Health Care America, Inc.