SYNVEXIA- lidocaine hydrochloride and menthol patch
Sterling Knight Pharmaceuticals,LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Synvexia
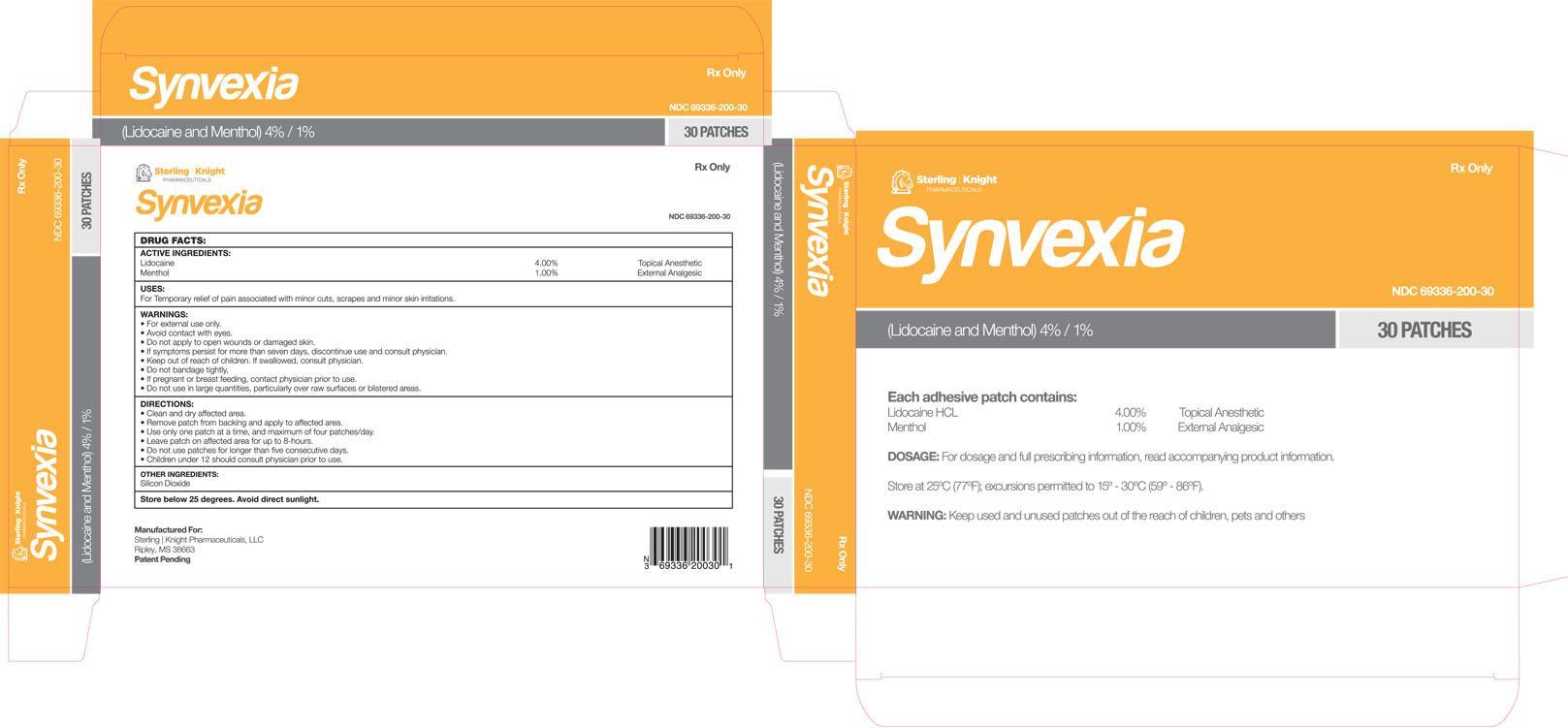
Warnings
For external use only
Avoid contact with eyes
Do not applly to open wounds or damaged skin
If symptoms persist for more than seven days, discontinue use and consult physician
If swallowed, consult physician
Do not bandage tightly
If pregnant or breast feeding, contact physician prior to use
Do not use in large quantities, particularly over raw surfaces or blistered areas
clean and dry affected area
remove patch from backing and apply to affected area
use only one patch at a time, and maximum of four patches/day
leave patch on affected area for up to 8 hours
do not use patches for longer than five consecutive days
children under 12 should consult physician prior to use
How Supplied
Use only one patch at a time, and maximum of four patches/day. leave patch on affected area for up to 8 hours
NDC:69336-200-05 5 in 1 BOX
NDC:69336-200-10 10 in 1 BOX
NDC:69336-200-15 15 in 1 BOX
NDC:69336-200-30 30 in 1 BOX
NDC:69336-200-60 60 in 1 BOX
| SYNVEXIA
lidocaine hydrochloride and menthol patch |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Sterling Knight Pharmaceuticals,LLC (079556942) |
| Registrant - Sterling Knight Pharmaceuticals,LLC (079556942) |