COMPOUND BENZOIN- compound benzoin tincture
Paddock Laboratories, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
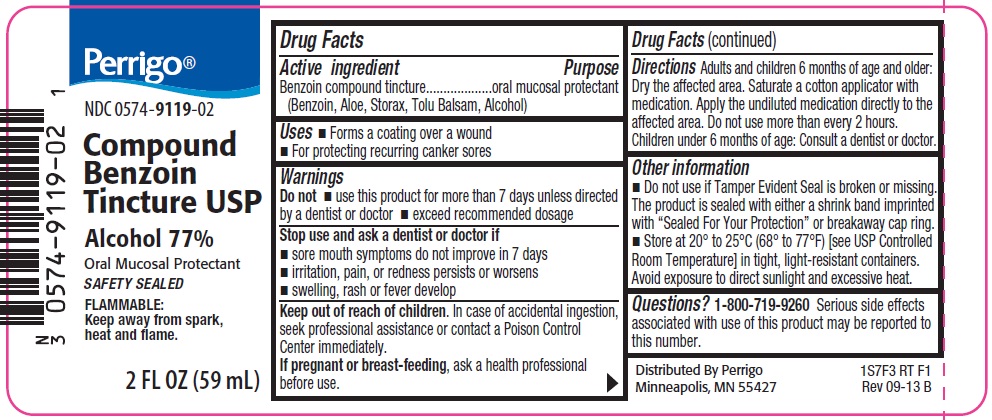
Perrigo Compound Benzoin Tincture USP
Stop use and ask a dentist or doctor if
- sore mouth symptoms do not improve in 7 days
- irritation, pain, or redness persists or worsens
- swelling, rash or fever develop
Keep out of reach of children.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Directions
Adults and children 6 months of age and older: Dry the affected area. Saturate a cotton applicator with medication. Apply the undiluted medication directly to the affected area. Do not use more than every 2 hours.
Children under 6 months of age: Consult a dentist or doctor.
Other information
- Do not use if Tamper Evident Seal is broken or missing. The product is sealed with either a shrink band imprinted with “Sealed for Your Protection” or breakaway cap ring.
- Store at 20 o to 25 oC (68 o to 77 oF) [see USP Controlled Room Temperature] in tight, light-resistant containers.
Avoid exposure to direct sunlight and excessive heat.
| COMPOUND BENZOIN
compound benzoin tincture |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Paddock Laboratories, LLC (967694121) |
| Registrant - Humco Holding Group, Inc. (825672884) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Humco Holding Group, Inc. | 825672884 | manufacture(0574-9119) , analysis(0574-9119) , pack(0574-9119) , label(0574-9119) | |
Revised: 11/2018
Document Id: 7a3e1208-8e8c-2ba4-e053-2991aa0af5bd
Set id: 8c08a567-d7ee-4f8b-847e-00dbeb6decdb
Version: 3
Effective Time: 20181109
Paddock Laboratories, LLC