Label: LIDOCAINE ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 71589-001-30, 71589-001-35, 71589-001-50 - Packager: Aleor Dermaceuticals Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 31, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
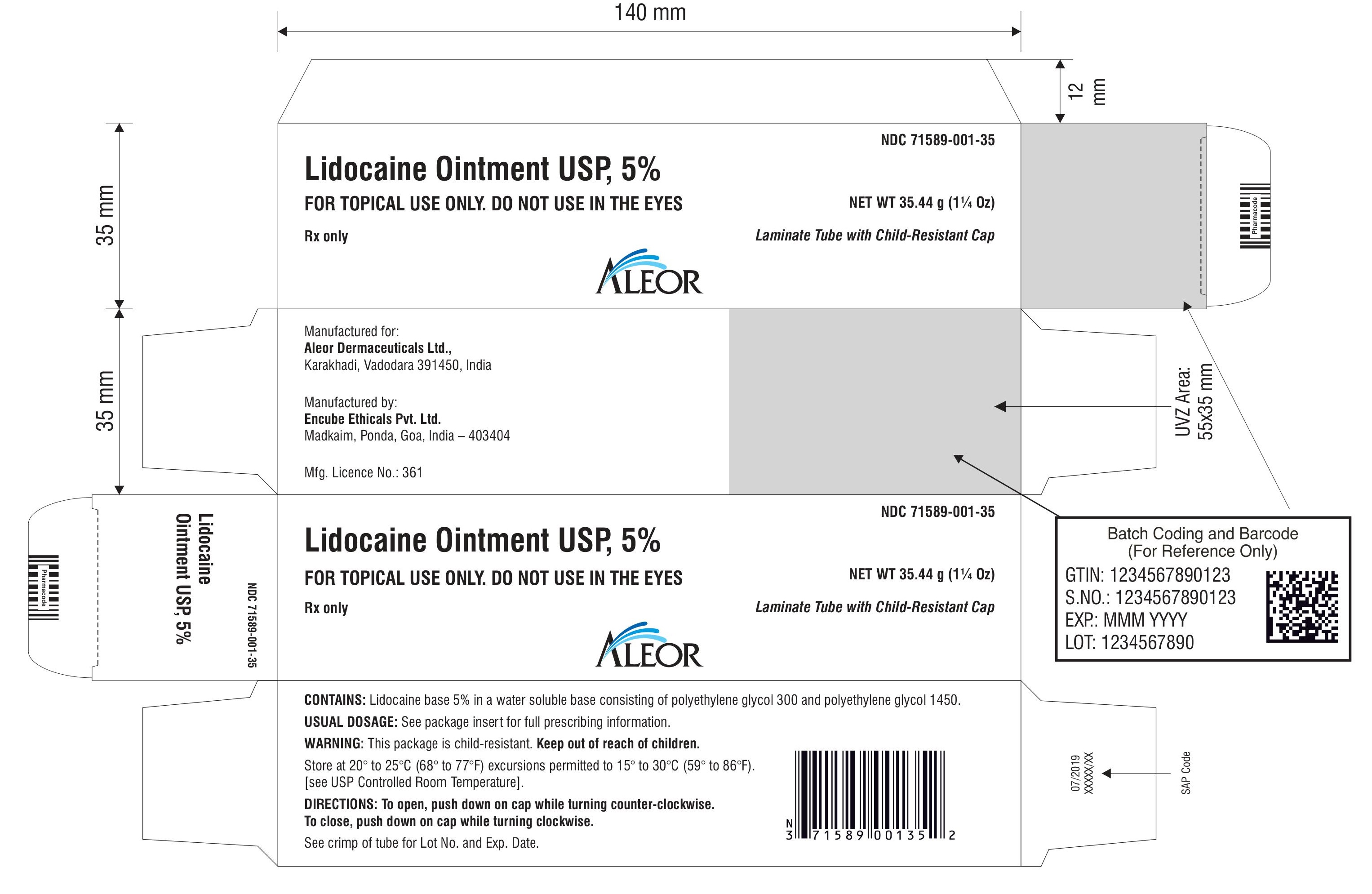
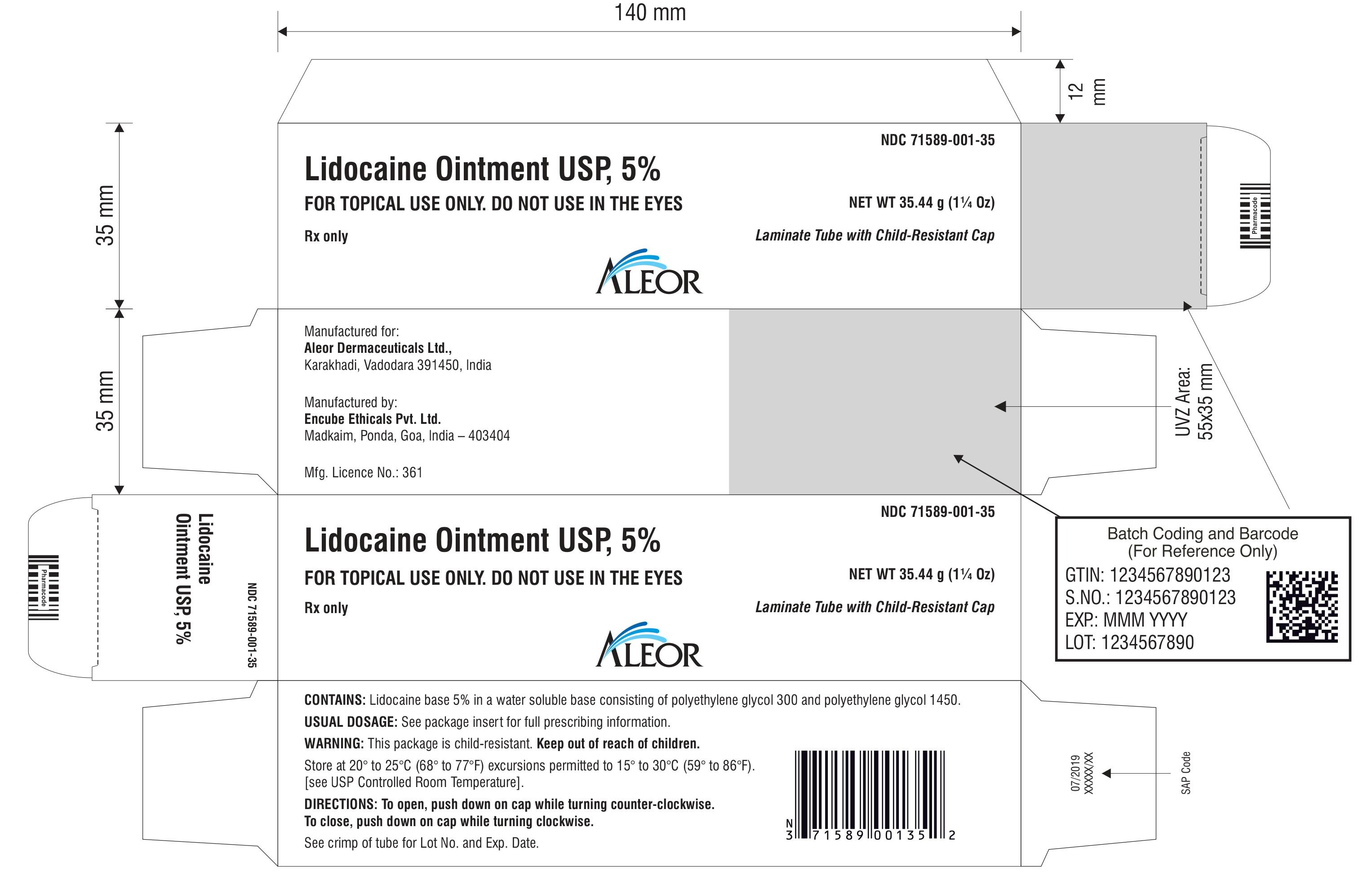
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Rx only
Lidocaine Ointment USP, 5%
Laminate Tube with Child-Resistant Cap
FOR TOPICAL USE ONLY. DO NOT USE IN THE EYES
NET WT 30 g

Rx only
Lidocaine Ointment USP, 5%
Laminate Tube with Child-Resistant Cap
FOR TOPICAL USE ONLY. DO NOT USE IN THE EYES
NET WT 35.44 g (1¼ Oz)
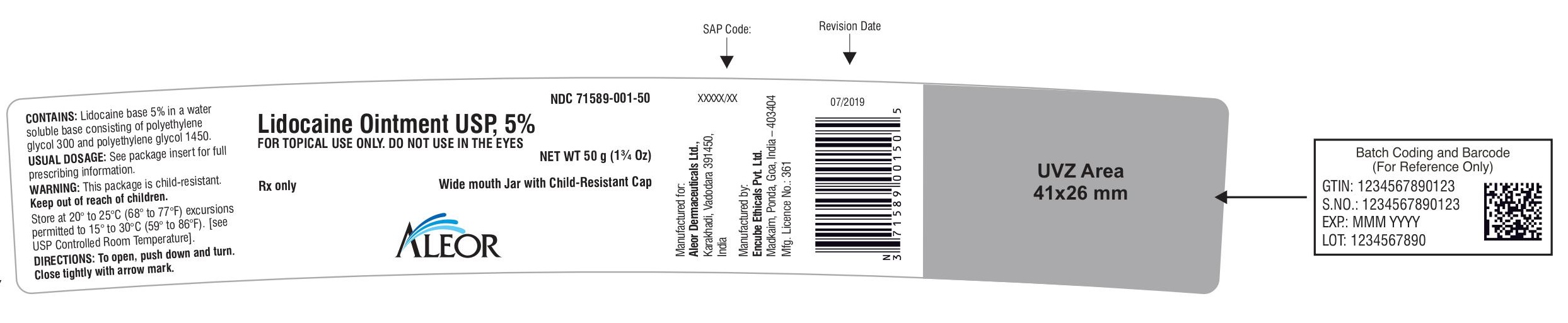
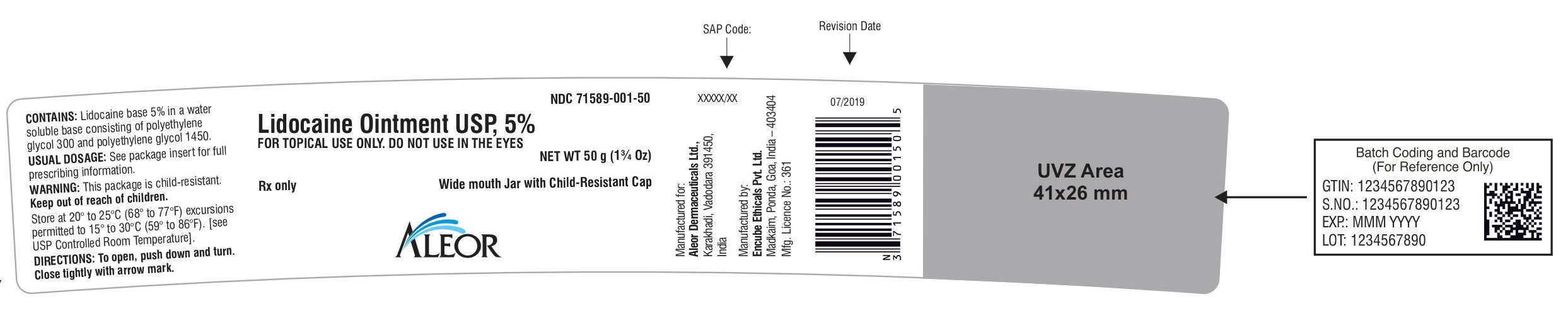
Rx only
Lidocaine Ointment USP, 5%
Wide mouth Jar with Child-Resistant Cap
FOR TOPICAL USE ONLY. DO NOT USE IN THE EYES
NET WT 50 g (1¾ Oz)
-
INGREDIENTS AND APPEARANCE
LIDOCAINE
lidocaine ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71589-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 300 (UNII: 5655G9Y8AQ) POLYETHYLENE GLYCOL 1450 (UNII: OJ4Z5Z32L4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71589-001-30 1 in 1 CARTON 11/30/2018 1 30 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:71589-001-35 1 in 1 CARTON 11/30/2018 2 35.44 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:71589-001-50 50 g in 1 JAR; Type 0: Not a Combination Product 11/30/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211469 11/30/2018 Labeler - Aleor Dermaceuticals Limited (871411532) Registrant - Aleor Dermaceuticals Limited (871411532) Establishment Name Address ID/FEI Business Operations Encube Ethicals Pvt. Ltd. 725076298 MANUFACTURE(71589-001)