Label: JUSTICE BERRY SCENTED ANTI-BACTERIAL HAND SANITIZER- alcohol gel
- NDC Code(s): 60637-219-30
- Packager: Tween Brands, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 25, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
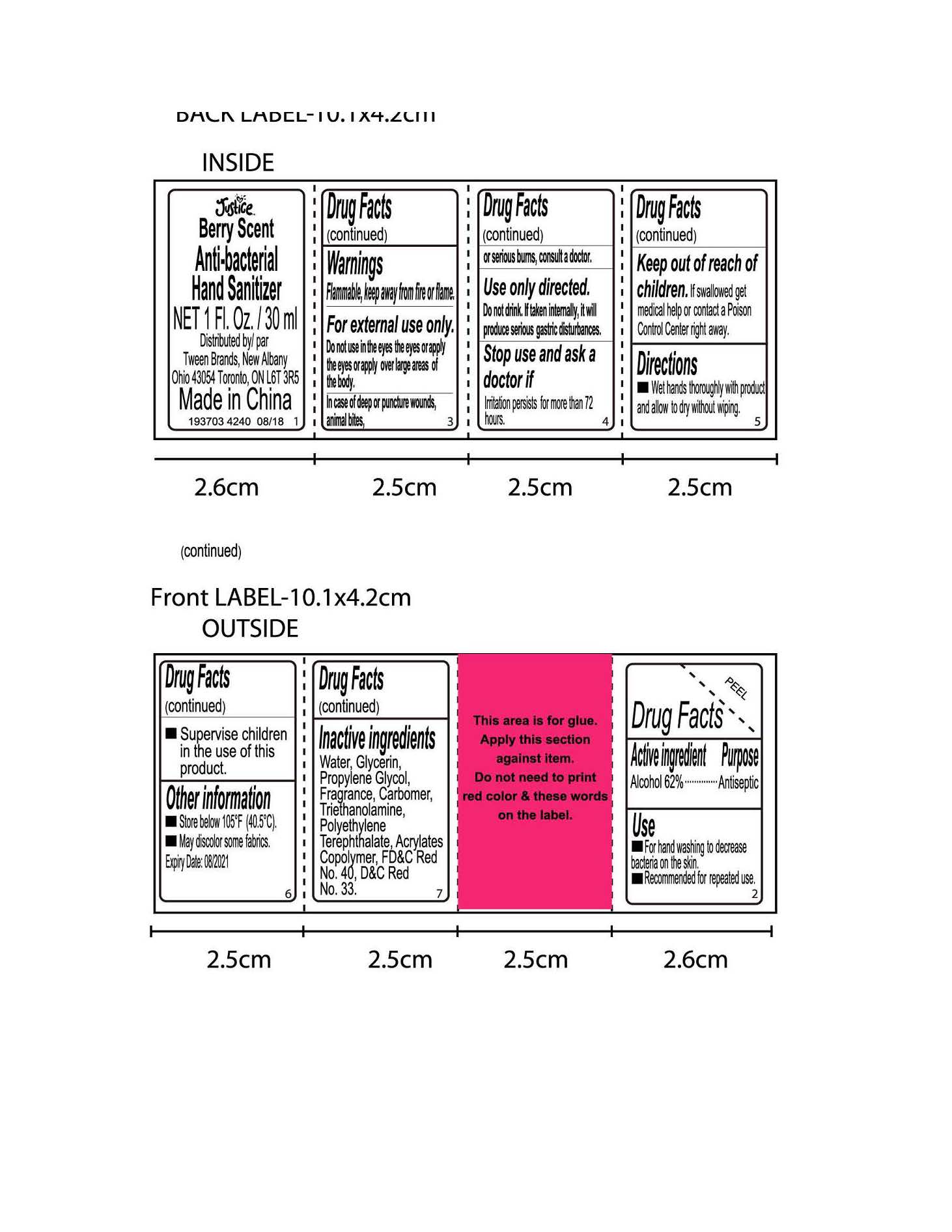
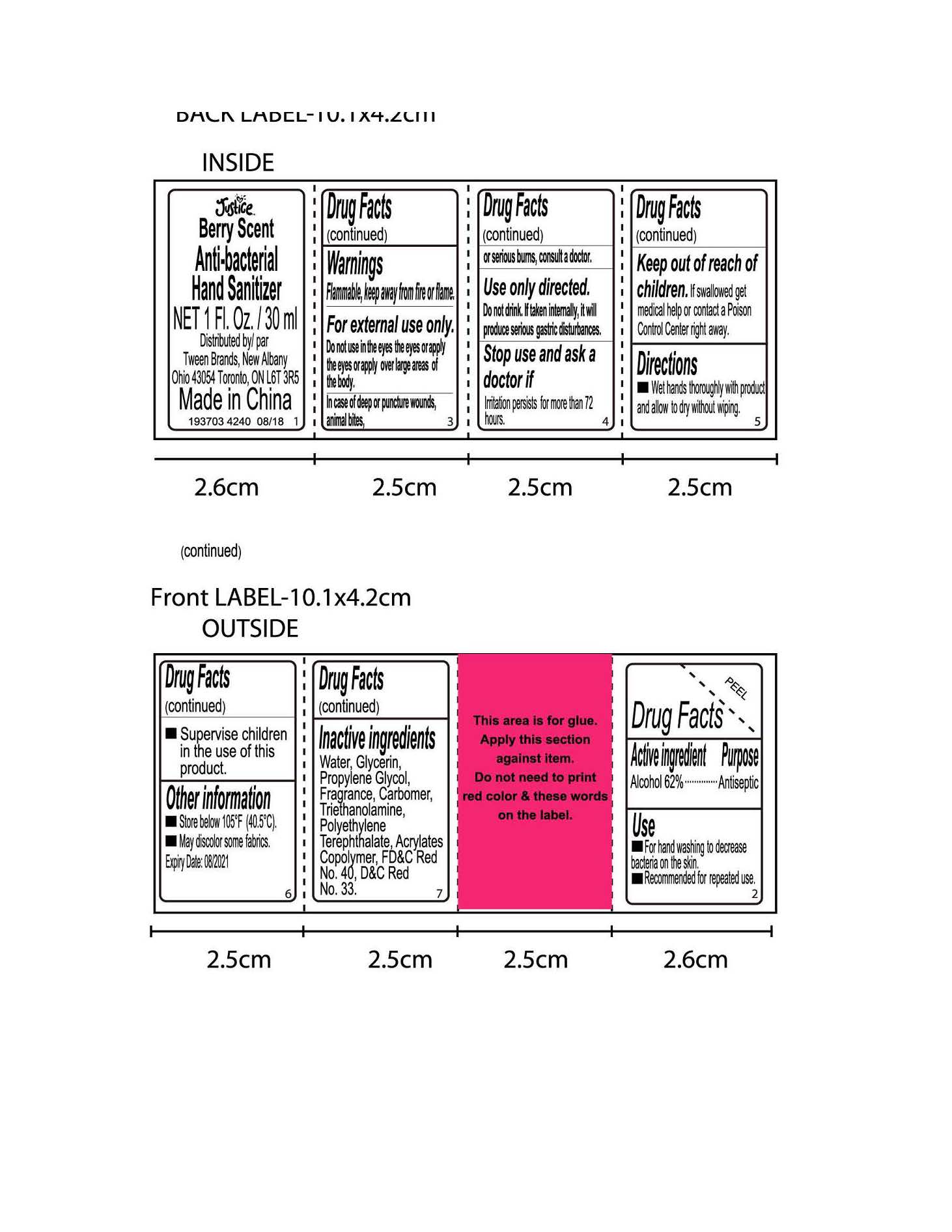
- Active Ingredient
- Purpose
- Use
- Directions
-
Warnings
Flammable, keep away from fire or flame
For external use only. Do not use in the eyes the eyes or apply the eyes or apply over large areas of the body
In case of deep or puncture wounds, animal bites, or serious burns, consult a doctor
Use only directed. Do not drink. If taken internally, it will produce serious gastric disturbance
Stop use and ask a doctor if irritation persists for more than 72 hours
- Inactive ingredients
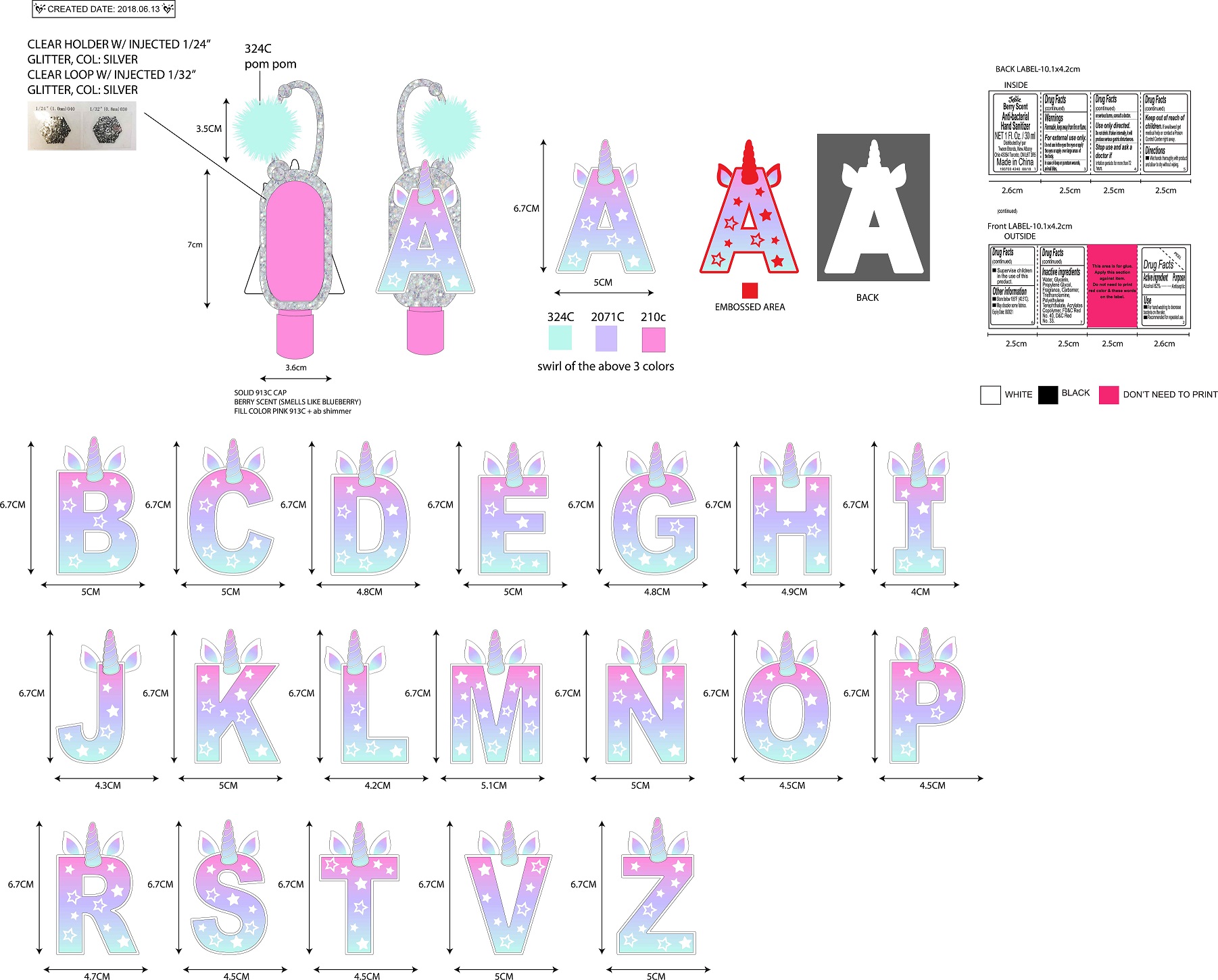
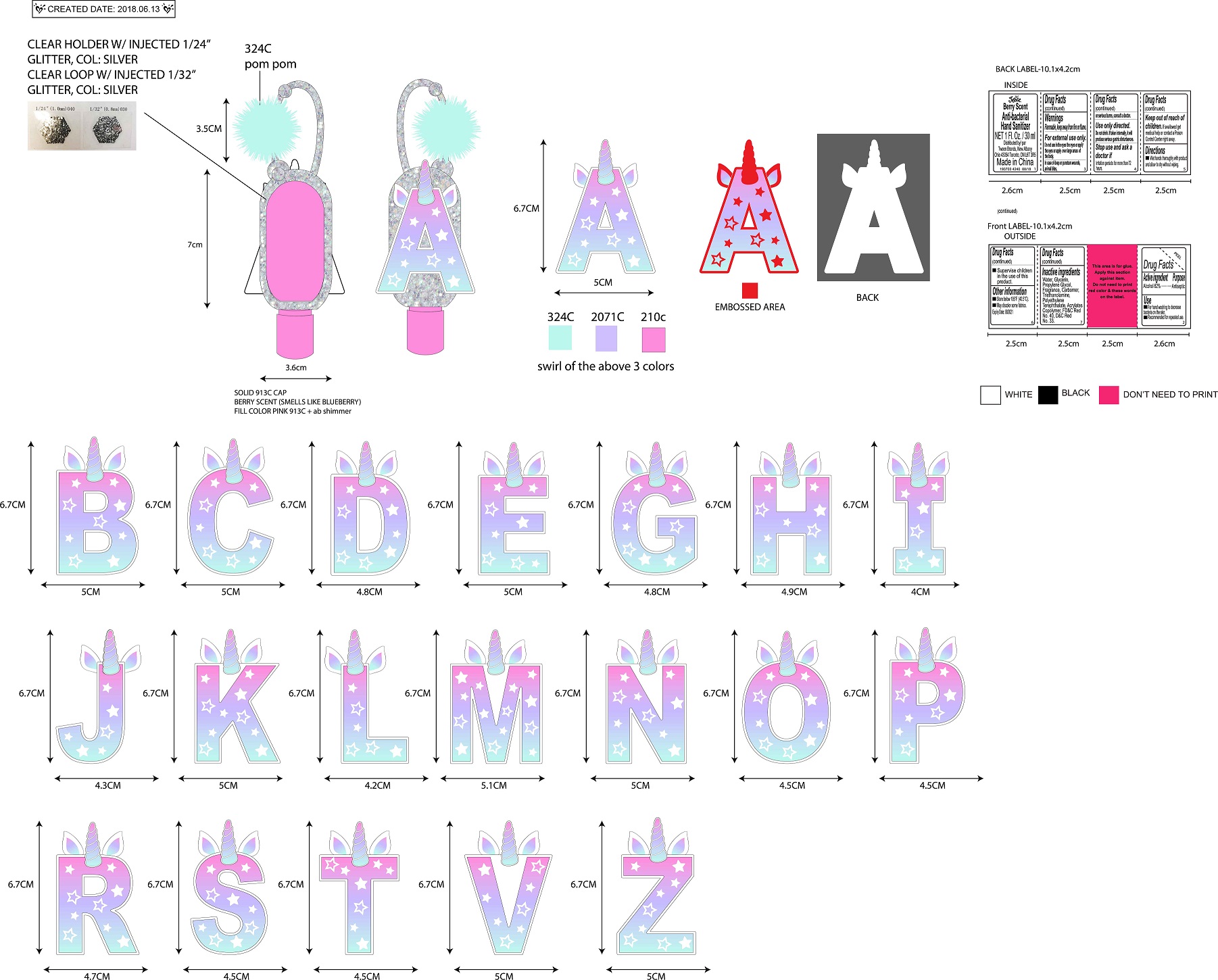
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
JUSTICE BERRY SCENTED ANTI-BACTERIAL HAND SANITIZER
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60637-219 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 620 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) 2,4-D-TROLAMINE (UNII: E6SLK39VZN) POLYETHYLENE TEREPHTHALATE (INTRINSIC VISCOSITY 0.40-0.70) (UNII: Y78AJD3DED) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) FD&C RED NO. 40 (UNII: WZB9127XOA) D&C RED NO. 33 (UNII: 9DBA0SBB0L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60637-219-30 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/20/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 06/20/2018 Labeler - Tween Brands, Inc. (965758188) Establishment Name Address ID/FEI Business Operations Bath Concept Cosmetics (Dongguan) Co., Ltd 529623933 manufacture(60637-219)