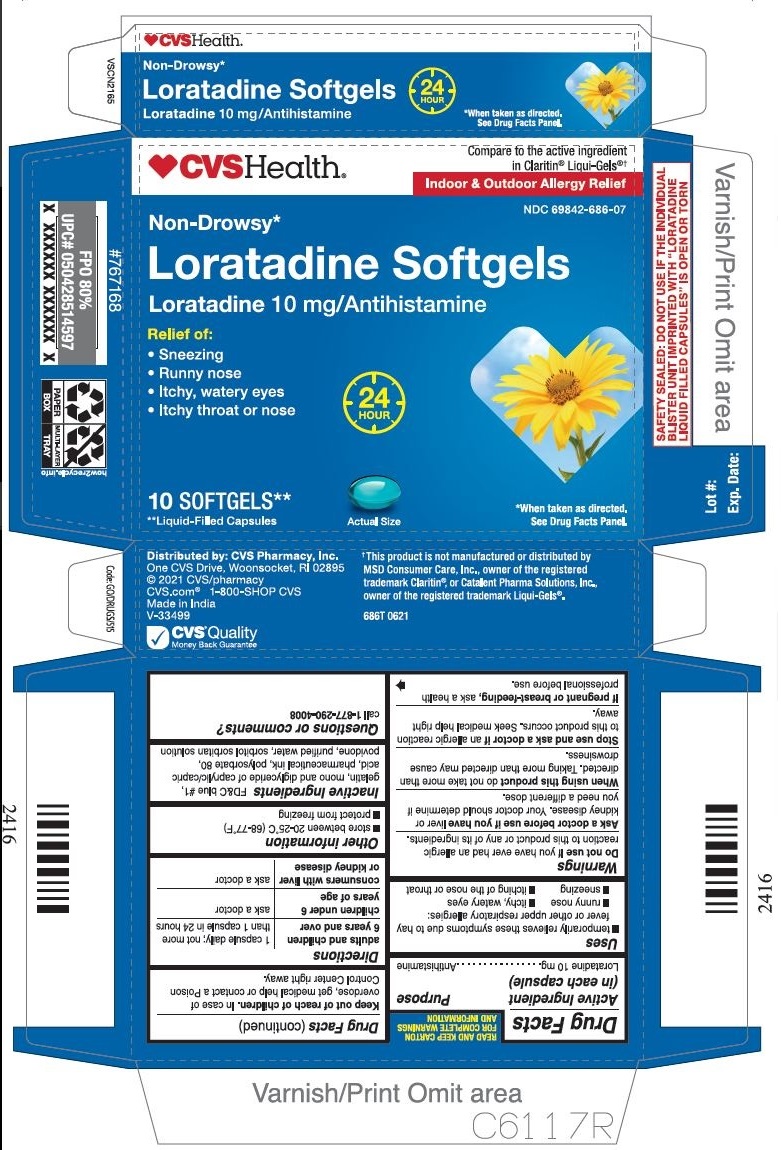
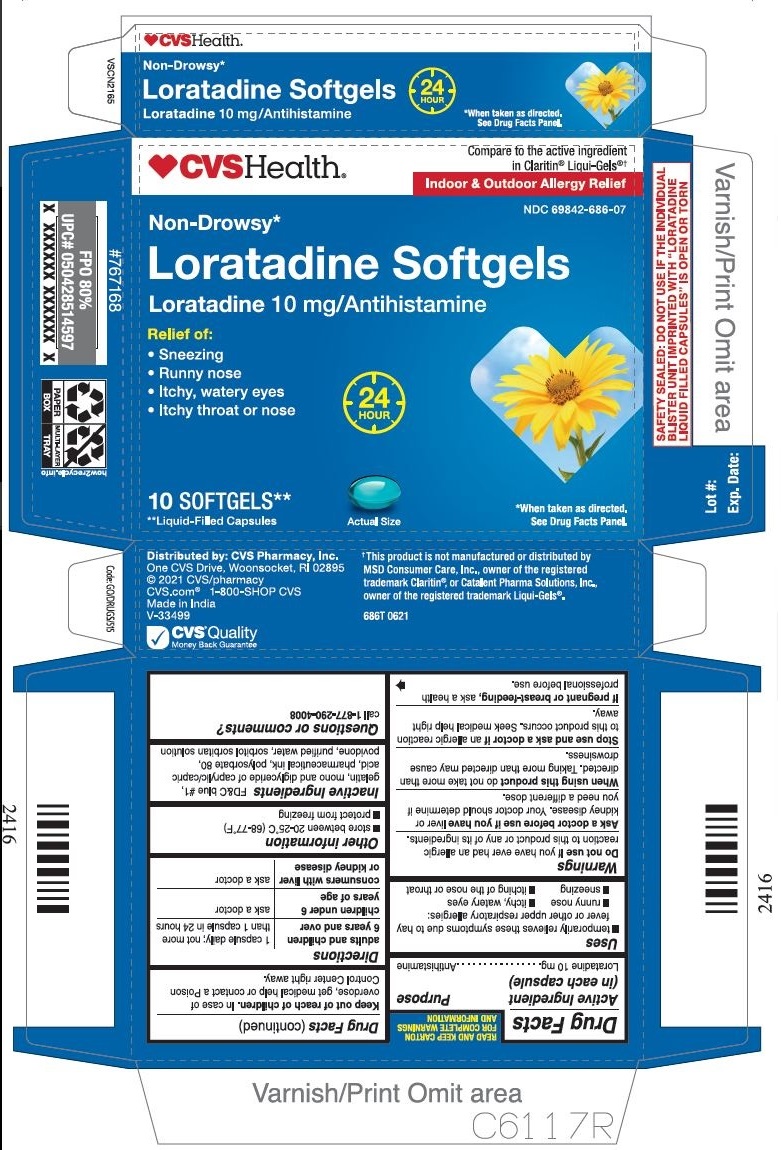
Label: LORATADINE capsule, liquid filled
- NDC Code(s): 69842-686-07, 69842-686-30, 69842-686-60
- Packager: CVS HEALTH CORP
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 11, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LORATADINE
loratadine capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-686 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POVIDONE (UNII: FZ989GH94E) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) CAPRYLIC/CAPRIC MONO/DIGLYCERIDES (UNII: U72Q2I8C85) Product Characteristics Color blue Score no score Shape OVAL Size 3mm Flavor Imprint Code 21 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-686-07 1 in 1 CARTON 01/31/2017 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:69842-686-30 3 in 1 CARTON 01/31/2017 2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:69842-686-60 6 in 1 CARTON 01/31/2017 3 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206214 01/31/2017 Labeler - CVS HEALTH CORP (062312574) Registrant - TIME CAP LABORATORIES, INC (037052099) Establishment Name Address ID/FEI Business Operations MARKSANS PHARMA LIMITED 925822975 manufacture(69842-686)