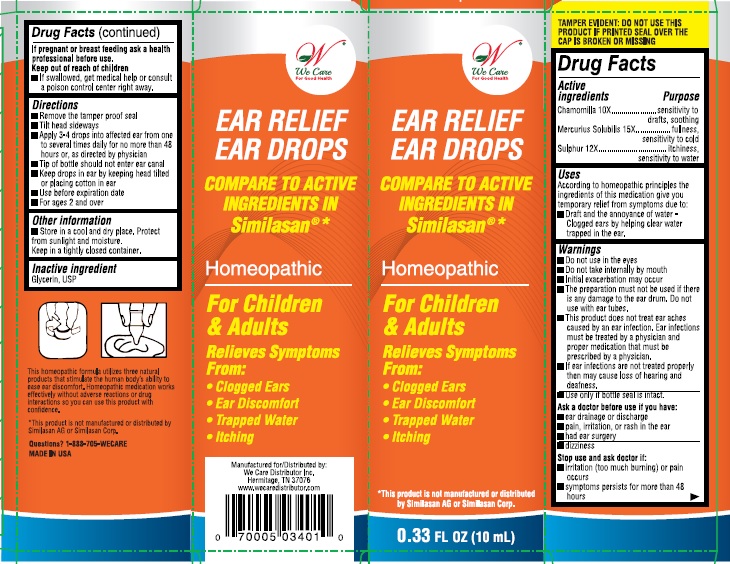
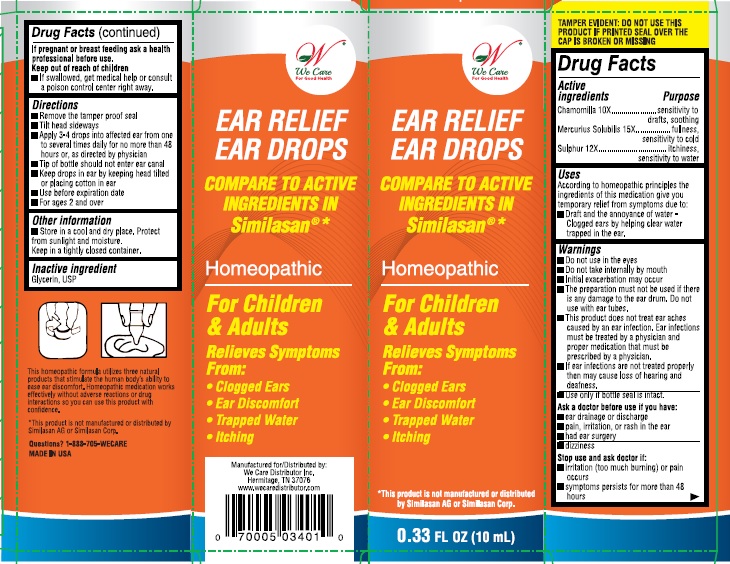
Label: EAR RELIEF EAR DROPS- chamomilla, mercurius solubilis, and sulphur solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 70005-034-01 - Packager: We Care Distributor Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated September 23, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Active ingredient
- Purpose
- Active ingredient
- Purpose
- Uses:
-
Warnings:
- Do not use in the eyes.
- Do not take internally by mouth
- Initial exacerbation may occur.
- The preparation must not be used if there is any damage to the ear drum, do not use with ear tubes.
- This product does not treat ear aches caused by an ear infection. Ear infections must be treated by a physician and proper medication that must be prescribed by a physician.
- If ear infections are not treated properly then may cause loss of hearing and deafness.
- Use only if bottle seal in intact.
- Ask a doctor before use if you have:
- Stop use and ask a doctor if:
- If pregnant or breast feeding;
- Keep out of reach of children.
-
Directions:
- Remove tamper proof seal
- Tilt head sideways
- Apply 3-4 drops into affected ear from one to several times daily for no more than 48 hours, or as directed by a physician
- Tip of the bottle should not enter ear canal
- Keep drops in ear by keeping head tilted or placing cotton in ear.
- Use before expiration date
- For ages 2 and over
- Other information:
- Inactive ingredient:
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EAR RELIEF EAR DROPS
chamomilla, mercurius solubilis, and sulphur solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70005-034 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHAMOMILE (UNII: FGL3685T2X) (CHAMOMILE - UNII:FGL3685T2X) CHAMOMILE 10 [hp_X] in 10 mL MERCURIUS SOLUBILIS (UNII: 324Y4038G2) (MERCURIUS SOLUBILIS - UNII:324Y4038G2) MERCURIUS SOLUBILIS 15 [hp_X] in 10 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 12 [hp_X] in 10 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70005-034-01 1 in 1 BOX 09/23/2016 1 10 mL in 1 BOTTLE, DROPPER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 09/23/2016 Labeler - We Care Distributor Inc. (079832998) Establishment Name Address ID/FEI Business Operations PURINE PHARMA LLC 019950491 manufacture(70005-034)