MY-SHIELD SANITIZING- benzalkonium chloride liquid
ESC Brands LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
My-Shield Sanitizing Soap
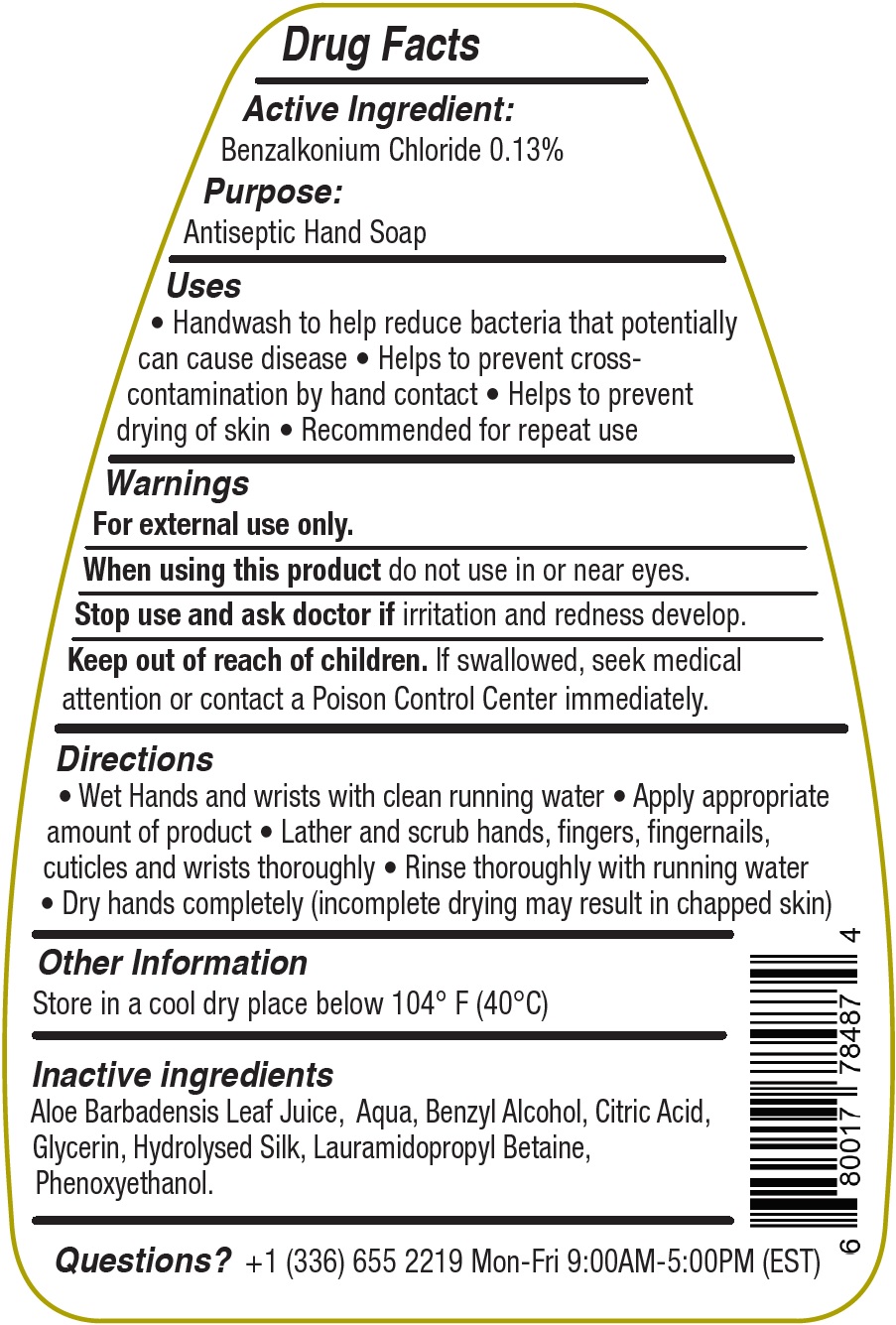
Uses
• Handwash to help reduce bacteria that potentially can cause disease • Helps to prevent crosscontamination by hand contact • Helps to prevent drying of skin • Recommended for repeat use
Directions
• Wet Hands and wrists with clean running water • Apply appropriate amount of product • Lather and scrub hands, fingers, fingernails, cuticles and wrists thoroughly • Rinse thoroughly with running water • Dry hands completely (incomplete drying may result in chapped skin)
| MY-SHIELD SANITIZING
benzalkonium chloride liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - ESC Brands LLC (202621850) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Filltech USA, LLC | 926433855 | manufacture(71884-003) | |
Revised: 10/2021
Document Id: cdc551cf-977e-0f18-e053-2a95a90aeb0b
Set id: 8858dcba-92d5-4895-ac07-dd73185d1094
Version: 4
Effective Time: 20211007
ESC Brands LLC