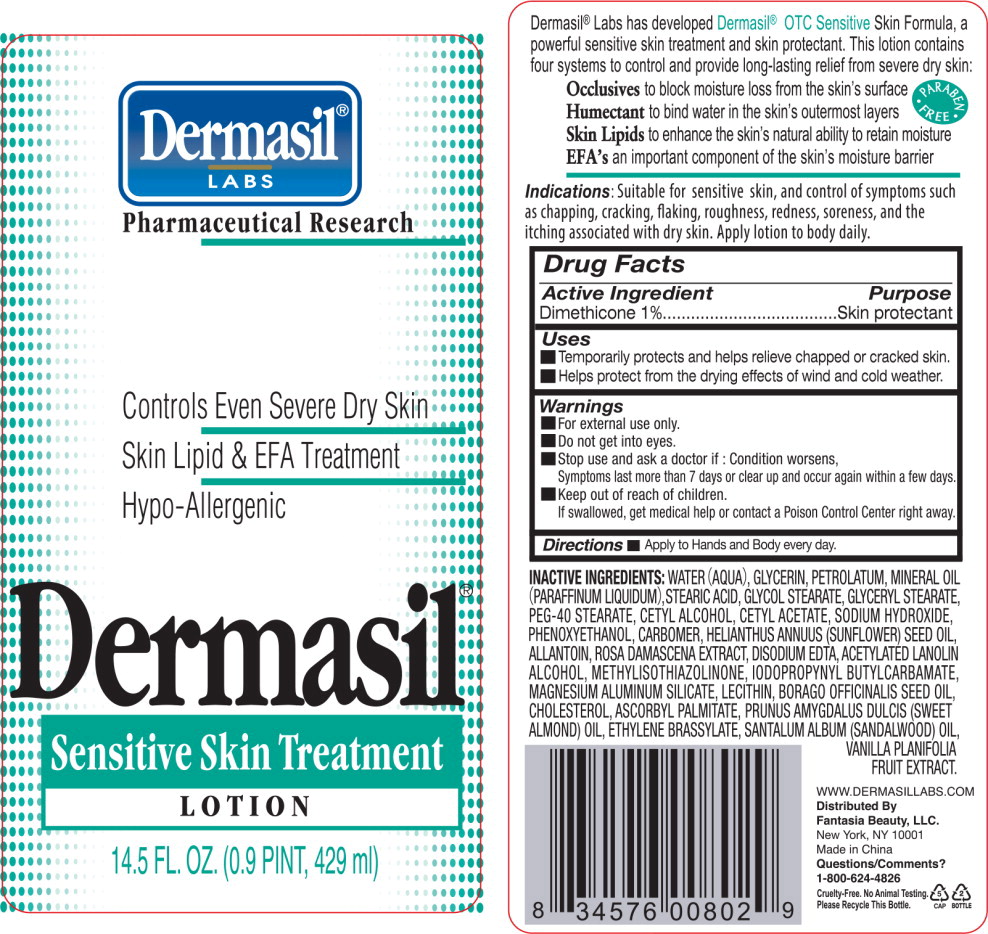
DERMASIL SENSITIVE- dimethicone lotion
Fantasia Beauty LLC
----------
Drug Facts
Uses
- Temporarily protects and helps relieve chapped or cracked skin.
- Helps protect from the drying effects of wind and cold weather.
Warnings
- For external use only.
- Do not get into eyes.
INACTIVE INGREDIENTS:
WATER (AQUA), GLYCERIN, PETROLATUM, MINERAL OIL (PARAFFINUM LIQUIDUM), STEARIC ACID, GLYCOL STEARATE, GLYCERYL STEARATE, PEG-40 STEARATE, CETYL ALCOHOL, CETYL ACETATE, SODIUM HYDROXIDE, PHENOXYETHANOL, CARBOMER, HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL, ALLANTOIN, ROSA DAMASCENA EXTRACT, DISODIUM EDTA, ACETYLATED LANOLIN ALCOHOL, METHYLISOTHIAZOLINONE, IODOPROPYNYL BUTYLCARBAMATE, MAGNESIUM ALUMINUM SILICATE, LECITHIN, BORAGO OFFICINALIS SEED OIL, CHOLESTEROL, ASCORBYL PALMITATE, PRUNUS AMYGDALUS DULCIS (SWEET ALMOND) OIL, ETHYLENE BRASSYLATE, SANTALUM ALBUM (SANDALWOOD) OIL, VANILLA PLANIFOLIA FRUIT EXTRACT.
Principal Display Panel - Dermasil Sensitive 7.25 Tube Label
Dermasil®
LABS
Pharmaceutical Research
Controls Even Sever Dry Skin
Skin Lipid & EFA Treatment
Hypo-Allergenic
Dermasil®
Sensitive Skin Treatment
LOTION
7.25 FL. OZ. (214 ml)
Principal Display Panel - Dermasil Sensitive 8 Tube Label
Dermasil®
LABS
Pharmaceutical Research
Controls Even Severe Dry Skin
Skin Lipid & EFA Treatment
Hypo-Allergenic
Dermasil®
Sensitive Skin Treatment
LOTION
8 FL OZ (236 ml)
Principal Display Panel - Dermasil Sensitive 8 Bottle Label
Dermasil®
LABS
Pharmaceutical Research
Controls Even Severe Dry Skin
Skin Lipid & EFA Treatment
Hypo-Allergenic
Dermasil®
Sensitive Skin Treatment
LOTION
8 FL OZ (1/2 PINT, 237 ml)
Principal Display Panel - Dermasil Sensitive 9.25 Tube Label
VALUE
SIZE
Dermasil®
LABS
Pharmaceutical Research
Controls Even Severe Dry Skin
Skin Lipid & EFA Treatment
Hypo-Allergenic
Dermasil®
Sensitive Skin Treatment
LOTION
9.25 FL. OZ. (274 ml)
Principal Display Panel - Dermasil Sensitive 10 Tube Label
Dermasil®
LABS
Pharmaceutical Research
Controls Even Severe Dry Skin
Skin Lipid & EFA Treatment
Hypo-Allergenic
Dermasil®
Sensitive Skin Treatment
LOTION
10 FL. OZ. (296 ml)
Principal Display Panel - Dermasil Sensitive 10 Bottle Label
Dermasil®
LABS
Pharmaceutical Research
Controls Even Severe Dry Skin
Skin Lipid & EFA Treatment
Hypo-Allergenic
Dermasil®
Sensitive Skin Treatment
LOTION
10 FL. OZ. (3/5 PINT, 296 ml)
| DERMASIL SENSITIVE
dimethicone lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Fantasia Beauty LLC (037273190) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Guangdong Kingkey Fine Chemical Co., Ltd. | 545349263 | MANUFACTURE(76184-0102) | |